Abstract
Despite new treatment modalities, the clinical outcome in a substantial number of patients with multiple myeloma (MM) has yet to be improved. Antibody-based targeted therapies for myeloma patients could make use of the HM1.24 antigen (CD317), a surface molecule overexpressed on malignant plasma cells and efficiently internalized. Here, a novel immunotoxin, HM1.24-ETA′, is described. HM1.24-ETA′ was generated by genetic fusion of a CD317-specific single-chain Fv (scFv) antibody and a truncated variant of Pseudomonas aeruginosa exotoxin A (ETA′). HM1.24-ETA′ inhibited growth of interleukin 6 (IL-6)-dependent and -independent myeloma cell lines. Half-maximal growth inhibition was observed at concentrations as low as 0.3 nM. Target cell killing occurred via induction of apoptosis and was unaffected in co-culture experiments with bone marrow stromal cells. HM1.24-ETA′ efficiently triggered apoptosis of freshly isolated/cryopreserved cells of patients with plasma cell leukemia and MM and was active in a preclinical severe combined immunodeficiency (SCID) mouse xenograft model. Importantly, HM1.24-ETA′ was not cytotoxic against CD317-positive cells from healthy tissue (monocytes, human umbilical vein endothelial cells). These results indicate that CD317 may represent a promising target structure for specific and efficient immunotoxin therapy for patients with plasma cell tumors.
Similar content being viewed by others
Introduction
Continuous progress has been made in the treatment of multiple myeloma (MM), but for a substantial number of patients MM is still difficult to control in the long term.1 Antibody-based therapies frequently used in lymphoma may also represent a promising strategy in MM, but few target antigens specific for malignant plasma cells could be identified so far.2 Yet, antibodies against different target antigens in MM are in clinical and preclinical development, including CD317.3, 4, 5, 6, 7
Unconjugated antibodies depend in their efficacy on effector functions such as induction of apoptosis, recruitment of immune effector cells or the complement system. Furthermore, antibody activity may be critically affected by polymorphisms in FcγR genes.8 Especially in patients with suppressed immune system or substantial tumor load, cytotoxic effector cell populations may be of limited effectiveness. Therefore, conjugation of cytotoxic compounds to antibodies is an interesting strategy and several myeloma-directed immunoconjugates have demonstrated potent activity in preclinical studies.9, 10, 11, 12, 13 Different strategies have been suggested for the conjugation of cytotoxic compounds to antibodies.14, 15 Chemical crosslinking of cytotoxic substances, as represented by the immunoconjugate gemtuzumab ozogamycin for acute myeloid leukemia, represents a useful approach. However, heterogeneous mixtures of unconjugated antibody and conjugates with varying amounts of crosslinked toxic compound may be obtained.16 In the past couple of years, progress has been made in the design of chemical crosslinkers and coupling chemistry, resulting in novel antibody–drug conjugates such as trastuzumab-emtansine (T-DM1) and Brentuximab vedotin (SGN-35) demonstrating impressive clinical responses.17, 18 This technology has also been used for the design of antibody–drug conjugates against surface receptors expressed on myeloma cells and the first clinical trials are on-going.11, 19
Genetic linkage of protein toxins, such as Pseudomonas exotoxin A, to single-chain fragment variable (scFv) fragments or disulfide stabilized Fv fragments (Fv) may represent an alternative to chemical crosslinking. Several reports demonstrated potent antitumor activity in vitro and in vivo with immunotoxins targeting tumor-associated antigens. The respective scFvs or Fvs were genetically fused to a truncated version of Pseudomonas exotoxin A (ETA′).20, 21, 22 An ETA′-based immunotoxin directed against CD22 (BL22) showed potent therapeutic activity in patients with hairy cell leukemia in a phase II clinical trial.23, 24
Besides tumor specificity, the internalization capacity of the targeted receptor and the specific epitope recognized by the targeting antibody critically determine the efficacy of immunoconjugates and immunotoxins.21, 25 Therefore, surface receptors with a high turnover and a high internalization rate are most promising to deliver cytotoxic compounds. The CD317 antigen may represent such an interesting target molecule. CD317 has been identified as a type II transmembrane protein of unusual topology that exists as disulfide-bonded dimer located in lipid rafts.26 It is detected on terminally differentiated B cells and is overexpressed on malignant plasma cells and some other tumor types like lung cancer and glioblastoma.27, 28, 29, 30 Although CD317 expression has originally been reported low or not detectable on most normal human tissues, a recent report suggested a less restricted expression pattern but the mechanisms of its regulation have yet to be clarified.27, 31
Here, a fusion protein consisting of a CD317-specific scFv and a truncated variant of Pseudomonas aeruginosa exotoxin A was generated. This novel immunotoxin, HM1.24-ETA′, showed cytotoxic activity in MM cells in vitro as well as in vivo and may represent an interesting approach for the treatment of MM.
Materials and methods
Isolation of primary patient MM cells
Fresh plasma cell leukemia and MM cells were isolated from blood or bone marrow aspirates drawn from patients after obtaining informed consent in accordance with the Declaration of Helsinki. Briefly, citrate- or heparin-anticoagulated blood or bone marrow was layered over a discontinuous gradient consisting of 70 and 62% Percoll (Biochrom, Berlin, Germany), respectively. After centrifugation, mononuclear cells (MNC) were collected from the serum/Percoll interface. CD138+ cells were enriched from MNC using CD138 MicroBeads (Miltenyi, Bergisch Gladbach, Germany) according to the manufacturer’s protocols. The cells were either used directly for the cytotoxicity assays or cryopreserved before further analysis. Experiments reported here were approved by the Ethics Committee of the Christian-Albrechts-University (Kiel, Germany).
Isolation of monocytes from healthy donors
Monocytes were enriched from MNC of healthy donors by magnetic activated cell sorting using ‘Monocyte Isolation Kit II’ (Miltenyi) according to the manufacturer’s protocol.
Cell lines
RPMI-8226, L363, Jurkat, Raji and CEM cells were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany). JK-6 and INA-6 cells were established in our laboratory.32, 33 All cell lines were cultured in RPMI-1640-Glutamax-I medium (Invitrogen, Karlsruhe, Germany) containing 10% fetal calf serum, penicillin and streptomycin (R10+). Medium of the interleukin 6 (IL-6)-dependent cell line INA-6 was supplemented with recombinant IL-6 (2.5 ng/ml; Biosource, Camarillo, CA, USA). Human umbilical vein endothelial cells (HUVECs) were obtained from Lonza (Basel, Switzerland) and cultivated in EGM medium with appropriate supplements (Lonza).
Generation of HM1.24-ETA′
The CD317-scFv was derived by SfiI digestion of pSEC-HM1.24xdsCD16,34 and ligated to SfiI-digested pet27b(+)-ETA′ carrying a variant of Pseudomonas exotoxin A codon optimized for the expression in Escherichia coli and resulting in pet27b(+)-HM1.24-ETA′.25 The cloned sequences were confirmed by Sanger sequencing.
Expression and purification of immunotoxins
The scFv-ETA′ fusion proteins were expressed under osmotic stress as previously described.35 Briefly, arctic express (DE3) RP cells were transformed with the expression construct and overnight cultures in 2xYT medium (Carl Roth GmbH, Karlsruhe, Germany) (supplemented with 20 μg/ml gentamycin, 75 μg/ml streptomycin, 50 μg/ml kanamycin and 1% glucose) were incubated at 37 °C with shaking. The culture was diluted to an OD600 below 0.1 and further incubated at 37 °C in a shaker incubator until OD600 reached 1.0. Osmotic stress was induced as described previously and the incubation temperature was reduced to 28 °C. After 1 h of incubation with agitation, the temperature was further reduced to 13 °C and IPTG (1 mM final concentration) was added. Induced cultures were harvested 16–20 h after induction. The bacterial pellet from 1 l culture was resuspended in 20 ml of extraction buffer (0.5 M sucrose, 0.1 M Tris, 1 mM EDTA, pH 8.0). The suspension was stirred for 30 min at 4 °C and sonicated with 4 bursts of 30 s followed by intervals of 30 s for cooling. The extract was cleared by centrifugation for 40 min at 20 000 g and 4 °C. The scFv-ETA′ fusion proteins were enriched by affinity chromatography using streptactin agarose matrix (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Aggregates and/or higher molecular weight contaminants were removed by gel filtration chromatography as described earlier.36
SDS–polyacrylamide gel electrophoresis and western blot analysis
SDS–polyacrylamide gel electrophoresis was performed using standard procedures. Proteins were stained with Coomassie brilliant blue R250 (Sigma-Aldrich Chemie GmbH, Munich, Germany) or transferred to Immuno-Blot PVDF membrane (Bio-Rad, Hercules, CA, USA). Recombinant immunotoxins were detected with mouse anti-penta-His antibody (Qiagen). Cleavage of poly(ADP-ribose) polymerase (PARP) was analyzed using whole-cell protein extracts prepared from 1 × 106 cells as previously published.37 Full-length PARP and its specific cleaved product were detected using mouse anti-human PARP antibody (Cell Signaling Technology, Danvers, MA, USA). Horseradish peroxidase-conjugated goat anti-mouse antibodies (Dianova, Hamburg, Germany) were used as secondary antibodies. Detection of bound antibody was performed with SuperSignal West Dura Extended Duration Substrate (Pierce, Rockford, IL, USA).
Capillary electrophoresis
Quantitative analysis of purified immunotoxins was performed using capillary electrophoresis on an Experion system (Bio-Rad) according to the manufacturer’s protocol.
Flow cytometric analyses
For immunofluorescence staining, 3 × 105 cells were washed in phosphate-buffered saline (PBS) supplemented with 1% bovine serum albumin (Sigma-Aldrich, Munich, Germany) and 0.1% sodium-azide (PBA buffer). To analyze immunotoxin binding, cells were incubated with HM1.24-ETA′ or control proteins at indicated concentrations for 30 min on ice. After washing twice with 500 μl PBA buffer, cells were stained with Alexa-Fluor-488 coupled mouse anti-penta-His antibody (Qiagen) or rabbit anti-exotoxin A polyclonal antibodies and fluorescein isothiocyanate (FITC)-labeled F(ab')2 fragments of polyclonal goat anti-rabbit antibodies (Sigma-Aldrich).
The purity of CD138+-enriched cells was analyzed by staining 3 × 105 cells with FITC-conjugated CD38 and PE-coupled CD138 antibodies (Beckman Coulter, Fullerton, CA, USA) according to the manufacturer’s protocol using appropriate controls.
CD317 expression on CD138+ cells was determined by staining 3 × 105 cells with a humanized HM1.24-IgG1 antibody (kindly provided by Chugai Pharmaceuticals Inc., Tokyo, Japan) or irrelevant IgG1 control, and FITC-coupled goat anti-human IgG F(ab')2 fragments (Beckman Coulter) as secondary antibody. Cells were analyzed on a flow cytometer (FC500, Beckman Coulter, Brea, CA, USA).
The surface expression level of CD317 was quantified by indirect immunofluorescence analysis using the QIFIKIT (Dako, Glostrup, Denmark) and mouse CD317 monoclonal antibody (clone 26F8, eBioscience, San Diego, CA, USA) as primary antibody according to the manufacturer’s protocol.
Measurement of cytotoxic effects of immunotoxin
For evaluation of cytotoxic effects of the immunotoxin, cells were seeded at 2 × 104 cells per 200 μl in 96-well plates. HM1.24-ETA′ or a control toxin was added at the indicated concentrations. After 3 days, vital cell mass was measured using colorimetric tetrazolium (MTT/MTS)-based assays (Cell Proliferation Kit I; Roche, Mannheim, Germany; Promega, Madison, WI, USA). For the detection of early stages of apoptosis and cell death, cells were seeded at 2 × 105 cells per ml in 24-well plates with increasing immunotoxin concentrations. For analyzing the kinetics of apoptosis induction, the immunotoxin was used at 100 ng/ml. Cells were stained with FITC-conjugated annexin V and 7-aminoactinomycin D (7-AAD; Beckman Coulter, Fullerton, CA, USA) according to the manufacturer’s protocol, and subsequently analyzed by flow cytometry. For blocking experiments, a 50-fold molar excess of parental antibody was added 30 min before adding immunotoxin.
MM/bone marrow stromal cells (BMSC) co-culture
BMSC were obtained from MNC isolated by ficoll density centrifugation of patient-derived bone marrow aspirates and subsequently cultured in R10+. For co-culture experiments, stromal cells were trypsinized and transferred to 96-well plates (0.5 × 104 cells/well), and MM cells (INA-6, 2 × 104 per well) were added the following day. Cells were treated with HM1.24–ETA′ at the concentrations indicated. For blocking experiments, the parental HM1.24-IgG1 antibody or a control antibody were added in molar excess. After 3 days of culture, stromal cell viability was analyzed by a colorimetric MTS-based assay (Promega). DNA synthesis of INA-6 cells under co-culture conditions was measured by [3H]-thymidine uptake. In brief, cells were pulsed with [3H]-thymidine (TdR; 1 μCi/well; specific activity, 5.0 Ci/mmol; Hartmann Analytic, Braunschweig, Germany) for 6 h. Subsequently, DNA was transferred onto glassfiber filters and counted in a β-scintillation counter (Perkin Elmer, Rodgau, Germany).
INA-6 xenograft tumor model
Seven-week-old female severe combined immunodeficiency (SCID) beige mice (Charles River, Sulzfeld, Germany) were maintained under pathogen-free conditions and injected intraperitoneally with 2.5 × 107 INA-6.Tu1 cells (these INA-6 cells have already been passaged in SCID mice) in 1 ml PBS. Starting on day 2 until day 12 every other day, and on days 20, 27 and 34, mice were treated intraperitoneally with HM1.24-ETA′ (total of 9 injections, 15 μg toxin each) or PBS as vehicle control. Tumor engraftment was monitored twice per week. Mice were killed when suffering from tumor burden, ascites, when paraplegia was observed or at the end of the experiment (day 125). All animal experiments and care were performed in accordance with legal regulations and the guidelines provided by the Federation of European Laboratory Animal Science Associations (FELASA) as well as approval of institutional authorities.
Statistical analyses and data processing
Graphical and statistical analyses were performed using GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA, USA). Curve fits were calculated assuming a sigmoidal dose response with variable slope. P-values were calculated using Student’s t-test, one- or two-way analysis of variance and the null hypothesis was rejected when P<0.05. Survival curves were analyzed using the log rank test.
Homology modeling
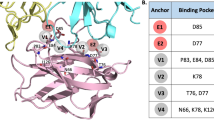
A homology model was calculated for HM1.24-ETA′ by modeling the scFv (template: 1H8N) and ETA′ (template: 1IKP) separately using the YASARA Structure software (YASARA Biosciences, Graz, Austria). The individual domains were manually fused using the YASARA Structure software. Ribbon drawings were performed using Discovery Studio 2.0 Visualize software (Accelrys Inc., San Diego, CA, USA).
Results
Generation and antigen-specific binding of HM1.24-ETA′
HM1.24-ETA′ was generated by fusing a CD317-specific scFv34 to a sequence optimized version of a truncated Pseudomonas exotoxin A lacking the receptor binding domain I (ETA′, Figure 1a). As controls, immunotoxins using a CD64-specific scFv or a CD7-specific scFv published earlier were used.22, 38 The immunotoxins were expressed in E. coli and purified from periplasmatic extracts by affinity chromatography and gelfiltration (Figures 1b and c). HM1.24-ETA′ specifically and dose dependently bound to CD317-positive L363 MM cells (Figure 1d). Binding of the immunotoxin was completely inhibited by preincubation of the cells with a molar excess of parental antibody, demonstrating that antigen specificity was not altered by fusing the toxin component (Figure 1e).
Design, purification and antigen-specific binding of HM1.24-ETA′. (a, left) Design of the recombinant immunotoxin HM1.24-ETA′. ETA′, truncated ETA fragment consisting of domains II and III of Pseudomonas exotoxin A; KDEL, endoplasmic reticulum retention motif; S, pelB secretion leader fused to STREP-II-6xHis-tag; SfiI, restriction site; T7, T7 promoter; VL, VH, variable regions of the light and heavy chain. (a, right) Calculated homology model of HM1.24-ETA′. (b) HM1.24-ETA′ purified by affinity chromatography was analyzed by capillary electrophoresis under reducing conditions. SP, system peaks. (c) Higher molecular mass contaminants were removed by gelfiltration. Graph shows reanalysis after final purification step. (d) Dose-dependent binding of HM1.24-ETA′ or control immunotoxin CD64-ETA′ was analyzed on L363 cells. Mean values±s.e.m. from three experiments are shown. (e) Competition binding experiments were performed to demonstrate specificity of HM1.24-ETA′ binding. Cells were stained with HM1.24-ETA′ and subjected to flow cytometry (black). Binding of HM1.24-ETA′ was inhibited with a molar excess of parental antibody (gray). Unstained cells (white). Data from one representative experiment out of three are shown.
Specific inhibition of myeloma cell line growth
To test whether the recombinant immunotoxin was biologically active, proliferation assays were performed. Proliferation of CD317-expressing myeloma cell lines L363, RPMI-8226, JK-6 and the IL-6-dependent plasmacytoma cell line INA-6 was significantly inhibited by HM1.24-ETA′. CD317-negative CEM (T-ALL) and Raji (Burkitt’s lymphoma) cells were not compromised (Figure 2a). A control immunotoxin did not show this inhibitory effect (Figure 2b). Half-maximal inhibition was achieved at concentrations of 30–50 ng/ml corresponding to ∼0.3–0.5 nM.
HM1.24-ETA′ specifically inhibits proliferation of MM cell lines. (a) Antigen-positive L363, INA-6, RPMI8226 and JK-6 and antigen-negative Raji and CEM cells were treated with a single dose of HM1.24-ETA′ (100 ng/ml). After 72 h, cell viability was analyzed using the MTT assay. White indicates untreated and black indicates treated with HM1.24-ETA′. (b) L363 and INA-6 cells were incubated with HM1.24-ETA′ (•) or CD64-ETA′ (□) at increasing toxin concentrations for 72 h. Vital cell mass was measured using the MTT assay. Data represent mean values±s.e.m. of three experiments. Significant differences with a P-value of P<0.05 are indicated (*). NS, not significant.
HM1.24-ETA′ is not cytotoxic against CD317-positive nonmalignant monocytes and HUVECs
Besides its expression on MM cells (Supplementary Figure 1), CD317 expression has also been detected on normal/nonmalignant tissue. For example, nonmalignant subepithelial plasma cells expressed CD317 as evidenced by immunohistochemistry of tissue sections (Figure 3a). In line with recently published data by Erikson et al.31 who reported ‘high level’ expression of CD317 on monocytes, flow cytometric analysis revealed significant staining of monocytes (Figure 3b). In addition, using immunohistochemistry staining and RNA profiling, Erikson et al.31 reported CD317 expression on different normal tissues, including endothelial cells from blood vessels. Importantly, although surface expression of CD317 was observed, HM1.24-ETA′ was not cytotoxic against primary monocytes and low-passage HUVEC cells (Figures 3b and c). Even at 10–20-fold higher concentrations required for complete inhibition of myeloma cell growth, HM1.24-ETA′ treatment did not show inhibitory effects on these nonmalignant cell types (Figures 3b and c). This unexpected finding may be related to a significantly lower antigen density expressed on healthy tissue vs MM cell lines (Supplementary Figure 1), differences in the kinetics of internalization, the cellular activation state or, as suggested in recent reports, because of different functions of CD317 in selected cell types.39, 40 In line with these findings, induction of low levels of surface-expressed CD317 on Jurkat T cell leukemia cells (CD317-inducible, CD7-positive) by interferon-α (IFN-α; Supplementary Figure 2) did not result in HM1.24-ETA′-triggered cell death (Supplementary Figure 2). Importantly, Jurkat cells were significantly inhibited by CD7-ETA′, a similarly constructed immunotoxin directed against CD7, demonstrating that lack of response to HM1.24-ETA′ was not because of a general resistance to ETA-based conjugates (Supplementary Figure 2).
Expression of CD317 on nonmalignant cells is not sufficient for induction of cell death by HM1.24-ETA′. (a) Analysis of CD317 expression by immunohistochemistry on nonmalignant and malignant plasma cells. (I) CD317 expression was demonstrated in nonmalignant subepithelial plasma cells (PC) but not in germinal center (GC). (II, III) Immunohistochemistry staining of an extramedullary pleomorphic plasma cell myeloma that shows a positive signal for CD317 ((II) hematoxylin and eosin staining, (III) staining with HM1.24-IgG1). Original magnification × 400 for all. Surface expression of CD317 on monocytes (b) and HUVEC (c) from healthy donors was analyzed by flow cytometry (black: HM1.24-IgG1, white: isotype control). In the absence or after treatment at varying concentrations of HM1.24-ETA′, viability of monocytes (▪) and HUVEC (□) was measured using the MTT assay. Data represent mean values±s.e.m. of three independent experiments with cells of different donors.
As the kinetics of internalization may impact the amount of delivered payload, the properties of monocytes and endothelial cells to internalize HM1.24 in comparison with plasma cell leukemia cells, a time-dependent clearance of a HM1.24-specific antibody bound to the cell surface, was analyzed by flow cytometry (Supplementary Figure 3A). After incubation at 37 °C, the amount of surface-bound CD317 antibody on monocytes was reduced to 60% compared with monocytes incubated at 4 °C (Supplementary Figures 3A and B). Therefore, monocytes displayed an internalization rate nearly comparable to the plasma cell leukemia cell line L363 (Supplementary Figures 3A and B). On endothelial cells (HUVEC), no differences in surface-bound antibody were detected after 4 h (Supplementary Figures 3A and B). This reduced internalization of antibody may explain the insusceptibility of CD317-positive endothelial cells (HUVECs) for the induction of apoptosis by the immunotoxin HM1.24-ETA′.
For CD317-positive monocytes displaying comparable internalization of CD317 without being affected by the delivery of HM1.24-ETA′ immunotoxin, other cellular characteristics may account for the ‘resistance’ of the cells. As the catalytic ETA′ domain abrogates protein synthesis, resting cells with low metabolic activity, for example isolated monocytes, may be less affected by this mode of action in contrast to malignant plasma cells that are highly active in protein synthesis.
In line with this hypothesis, G28–5 sFv-PE40, an immunotoxin targeting CD40, efficiently killed CD40-positive malignant B cells, whereas monocytes and endothelial cells expressing CD40 were resistant to the immunotoxin.41 Activation of these cell populations by IFN-γ (monocytes) or a combination of IFN-γ and tumor necrosis factor-α (endothelial cells) sensitized these cells for the action of the immunotoxin. To analyze the influence of IFN-γ-mediated activation of monocytes on their susceptibility to HM1.24-ETA′-induced apoptosis, isolated monocytes were treated with the immunotoxin for 3 days with or without IFN-γ treatment. Cell viability was reduced in a dose-dependent manner by HM1.24-ETA′ only in the presence of IFN-γ (Supplementary Figure 3C). Thus, cytokine-mediated activation of monocytes sensitized these cells for HM1.24-ETA′-induced cell death, whereas nonactivated monocytes were almost unaffected. Interestingly, the surface level of CD317 on monocytes was not altered by IFN-γ treatment (Supplementary Figure 3D). Therefore, IFN-γ-induced activation rather than altered surface expression levels of CD317 may account for the enhanced potency of the immunotoxin HM1.24-ETA′ on IFN-γ-activated monocytes.
Together, these data suggest that a certain extent of surface expression on healthy tissue may not necessarily result in toxic side effects against nonmalignant tissue and the metabolic state/activation status of a target cell may govern its susceptibility to immunotoxin action.
Antigen-specific induction of apoptosis by HM1.24-ETA′
To analyze whether the inhibition of MM cell growth induced by HM1.24-ETA′ was antigen-specific and occurred via induction of apoptosis, JK-6 cells were treated with a single dose of immunotoxin (100 ng/ml) with or without preincubation with a molar excess of parental antibody, HM1.24-IgG1 (kindly provided by Chugai Pharmaceuticals), or a control IgG1 antibody. After 24 h, ∼95% of cells treated with HM1.24-ETA′ alone or with HM1.24-ETA′ in the presence of an irrelevant IgG1 antibody were found to be annexin V or annexin V/7-AAD double positive (Figure 4a), indicating that cell death occurred via induction of apoptosis. Excess of the parental HM1.24-IgG1 antibody prevented induction of apoptosis, demonstrating that killing was antigen specific.
Antigen-specific induction of apoptosis by HM1.24-ETA′ is inhibited by parental HM1.24-IgG1 antibody. (a) JK-6 cells were incubated with a single dose of HM1.24-ETA′ (100 ng/ml) for 24 h and analyzed by annexin V/7-AAD staining and flow cytometry. Preincubation of the cells with a molar excess of the parental antibody HM1.24-IgG1, but not with an irrelevant IgG1, blocked induction of apoptosis. (b) Protein extracts of HM1.24-ETA′-treated JK-6 and Raji cells were subjected to SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and western blot analysis. Detection of cleaved PARP confirmed antigen-specific induction of apoptosis by HM1.24-ETA′. Data show one representative experiment out of three performed.
Cleavage of PARP was used as an independent parameter to verify the induction of apoptosis by HM1.24-ETA′. Western blot analyses of protein extracts of JK-6 cells treated with a single dose (0.5 μg/ml) of immunotoxin for 24 h show cleavage of PARP. In contrast, only a small amount of cleaved PARP was detectable in all cell lysates from both immunotoxin-treated antigen-negative Raji cells or cell lysates of untreated JK-6 cells. Preincubation of JK-6 cells with parental antibody HM1.24-IgG1 completely blocked induction of HM1.24-ETA′-medated apoptosis (Figure 4b).
Dose- and time-dependent induction of apoptosis by HM1.24-ETA′
To analyze dose-dependent induction of apoptosis, L363, JK-6, INA-6 and Raji were treated with varying toxin concentrations (Figure 5a). A single dose of 100 ng/ml of HM1.24-ETA′ induced apoptosis in almost all JK-6 and L363 cells within 24 h. Half-maximal induction of apoptosis occurred at concentrations as low as 20 and 30 ng/ml, respectively. In contrast, INA-6 cells were less sensitive to immunotoxin treatment, although INA-6 cells expressed higher levels of CD317 than JK-6 or L363 (Supplementary Figure 1), suggesting that additional cellular factors govern extent of immunotoxin-induced cell death.
Time- and dose-dependent induction of apoptosis in MM cell lines by HM1.24-ETA′. (a) Dose-dependent induction of apoptosis and cell death was analyzed by treatment of different CD317-positive MM cell lines (JK-6 ∇, INA-6 □, L363 ▪) and antigen-negative Raji ( × ); t=24 h. (b) Kinetics of HM1.24-ETA′-mediated apoptosis induction. JK-6 cells (∇), L363 (▪), INA-6 (□) and CD317-negative Raji ( × ) were incubated with a single dose of HM1.24-ETA′ (100 ng/ml). Percentage of annexin V-positive cells was determined by flow cytometry at different time points. Data represent mean values of two independent experiments.
To investigate the kinetics of apoptosis induction by HM1.24-ETA′, the cell lines were incubated with a single dose of toxin that was found to be sufficient to induce apoptosis within 24 h of treatment. Cells were analyzed by annexin V/7-AAD staining at different time points (Figure 5b). Half-maximal induction of apoptosis was detected after 6.5 h. Approximately 80% of JK-6 and L363 cells were found to be annexin V/7-AAD positive already after 8 h, indicating that toxin uptake and processing was fast and efficient. Raji cells that served as controls were unaffected by immunotoxin treatment (Figures 5a and b).
Stromal cells do not protect plasma cells from HM1.24-ETA′-induced apoptosis
BMSC have been reported to protect myeloma cells from the cytotoxic effects of therapeutic drugs.42 To address whether bone marrow stroma mediates resistance to HM1.24-ETA′, INA-6 cells were co-cultured with stromal cells obtained from patient bone marrow aspirates. Addition of a single dose of HM1.24-ETA′ (30 ng/ml) completely inhibited INA-6 proliferation in the presence of BMSC (Figure 6a), whereas the unconjugated HM1.24-IgG1 antibody had no inhibitory effect on myeloma cell growth. In contrast, the viability of BMSC was not affected, even at higher HM1.24-ETA′ concentrations up to 300 ng/ml (Figure 6b), indicating that HM1.24-ETA′ was directly active against myeloma cells and not via affecting stromal cell viability. HM1.24-ETA′-mediated killing during co-culture was antigen specific, as indicated by blocking experiments using the parental antibody. Together, these data indicate that bone marrow stroma has no protective activity against HM1.24-ETA′-mediated induction of apoptosis.
HM1.24-ETA′ inhibits myeloma cell growth in the presence of BMSC. (a) INA-6 cells were cultivated in the presence of BMSC. Inhibition of myeloma cell proliferation after treatment with HM1.24-ETA′ or HM1.24-IgG1 was analyzed by thymidine incorporation (note that BMSC do not show thymidine incorporation under the described assay conditions). (b) The effect of HM1.24-ETA′ and HM1.24-IgG1 antibody on stromal cell viability was analyzed by MTS assay. Data represent mean values±s.e.m. of four independent experiments using stroma cells from different donors.
HM1.24-ETA′ induces apoptosis in freshly isolated/cryopreserved plasma cell leukemia and MM cells
To investigate the cytotoxic activity of HM1.24-ETA′ on freshly isolated tumor cells, malignant plasma cells were enriched from MNC obtained from bone marrow aspirates, pleural effusion or peripheral blood samples of seven patients with MM or plasma cell leukemia (Supplementary Table 1). Purity of CD138+ selected plasma cells from patients was ∼95% (Figure 7a). Importantly, isolated CD138+ cells from all patients homogenously expressed CD317 on the cell surface (Figure 7a and Supplementary Figure 1), although at lower levels compared with MM cell lines (Supplementary Figure 1).
HM1.24-ETA′ induces apoptosis in malignant plasma cells isolated from patients with multiple myeloma or plasma cell leukemia. (a) Mononuclear cells from blood of a patient (p#1) with plasma cell leukemia were analyzed for expression of CD317 on CD138+/ CD38+ malignant plasma cells (black) and isotype control (gray). (b) CD138 magnetic activated cell sorting (MACS)-sorted primary tumor cells were treated with HM1.24-ETA′. Immunotoxin-induced apoptosis was measured by annexin V/7-AAD staining. Mean values of single experiments set up in duplicates are shown. (c) Primary tumor cells of two patients were incubated with a single dose of 100 ng/ml HM1.24-ETA′ for 24 h. Annexin V/7-AAD staining indicated that HM1.24-ETA′ induced apoptosis. The cytotoxic effect mediated by HM1.24-ETA′ is blocked by preincubation of the tumor cells with a molar excess of parental antibody HM1.24-IgG1 but not by an irrelevant antibody. (d, left) Comparison of untreated sample group with treated sample group. (d, right) The corresponding data with HM1.24-IgG1 blocking antibody or irrelevant blocking antibody. HM1.24-ETA′=100 ng/ml, 24 h treatment; •, untreated; ▪, HM1.24-ETA′ treatment; □, HM1.24-ETA′+irrelevant IgG1; ○, HM1.24-ETA′+HM1.24-IgG1.
HM1.24-ETA′ induced apoptosis in primary malignant plasma cells in a dose-dependent manner. Approximately 75% of plasma cells (patient no. 1) treated with a single dose of 1 μg/ml for 24 h were found to be in early apoptotic state or dead as measured by annexin V/7-AAD staining (half-maximal effective concentration: 250 ng/ml, Figure 7b). The percentage of apoptotic cells after treatment with 100 ng/ml of immunotoxin increased from 25% after 24 h to 47% after 72 h, showing time-dependent induction of apoptosis at low immunotoxin concentrations (Figure 7b).
To further confirm the potency of HM1.24-ETA′ on patient-derived tumor cells, primary malignant plasma cells of six additional patients (2–7) were subjected to immunotoxin treatment (Figures 7c and d). HM1.24-ETA′ induced antigen-dependent apoptosis in all samples investigated (Figures 7c and d). The percentage of apoptotic cells obtained after 24 h of treatment with a suboptimal single dose of HM1.24-ETA′ (100 ng/ml) was variable between the different patients, indicating varying sensitivity or kinetics of immunotoxin uptake. Blocking experiments further confirmed the antigen specificity of the immunotoxin (Figures 7c and d). When mean values were calculated from all experiments, significant killing was observed between the nontreated and HM1.24-ETA′-treated group (Figure 7d, left panel), or between the specifically blocked and irrelevantly blocked sample group (Figure 7d, right panel). Thus, the induction of antigen-dependent killing was demonstrated in a panel of samples (Figures 7c and d) that were derived from patients differing in disease stage and subtype, as well as cytogenetic abnormalities (Supplementary Table 1).
HM1.24-ETA′ inhibits INA-6 tumors in SCID mice
To evaluate whether HM1.24-ETA′ was also active in vivo, 2.5 × 107 INA-6 cells that demonstrated the lowest sensitivity to HM1.24-ETA′ treatment in vitro were injected intraperitoneally into 7-week-old SCID beige mice. Starting on day 2 after tumor cell injection, mice were treated with 9 doses of HM1.24-ETA′ (total dose: 135 μg) or vehicle (PBS). HM1.24-ETA′ prevented plasmacytoma engraftment after the observation time of 125 days in 8/10 mice treated with HM1.24-ETA′. The difference in survival of HM1.24-ETA′-treated vs control group reached statistical significance (Figure 8; P<0.02).
HM1.24-ETA′ prevents engraftment of INA-6 plasmacytoma cells in SCID mice. INA-6 cells were intraperitoneally injected in SCID beige mice and groups of 10 animals were treated with either HM1.24-ETA′ or vehicle control. A statistically significant difference in survival was observed between the two treatment groups (P<0.02, log rank test).
Discussion
The novel single-chain immunotoxin HM1.24-ETA′ targeting the CD317 antigen, a surface receptor overexpressed on malignant plasma cells, was designed to advance therapeutic options for patients with MM, and potent antimyeloma activity was demonstrated in vitro and in a preclinical in vivo model.
HM1.24-ETA′ specifically triggered apoptosis in malignant plasma cells at low nM concentrations, comparable to potent ETA-based immunotoxins, such as BL22 (CAT-3888).43 Interestingly, induction of apoptosis in cell lines was detected within a few hours after treatment, indicating that uptake and intracellular processing of the toxin occurred efficiently and rapidly. This may be because of CD317 involvement in cell signaling44 as well as in endocytosis.39, 45 Earlier studies suggested the transmembrane and GPI-linked protein CD317 is a highly internalized antigen that is not dramatically downmodulated after internalization. Even in the absence of ligand or antibody binding, CD317 is internalized from lipid rafts in a clathrin-dependent manner. It is mainly transported to recycling endosomes and/or the trans-Golgi network without being delivered to late endosomes or lysosomes for degradation. CD317 is located at the cell surface, but also exists in intracellular stores and shuttles between the trans-Golgi network and the cell surface.26, 39 Thus, an efficient ‘transport cycle’ for the immunotoxin of the receptor may account for the observed potency.26, 39
Besides these highly dynamic characteristics of CD317 in cells active in secretion or internalization, recent reports suggested that in polarized endothelial and epithelial cells, CD317 exhibits a more structural function. CD317 is engaged in adhesion of myeloid cells to CD317-expressing endothelial cells.46 In polarized epithelial cells a complex of RICH2 and CD317 is involved in the organization of the subapical actin cytoskeleton.40 In these cells no intracellular depots of CD317 were detectable, whereas CD317 was only internalized inefficiently. These reports are consistent with our surface retention studies of a CD317-specific antibody (Supplementary Figure 3). Clathrin-mediated endocytosis depends on the interaction of the AP2 adaptor complex and the corresponding binding motive within the cytoplasmic domain of CD317.47 This motive shared by the adaptors AP2 and RICH2 may not be accessible if occupied by RICH2, thereby preventing CD317 internalization. This specific dependency on different adaptor complexes may in part account for the differential susceptibility observed for CD317-positive monocytes and endothelial cells compared with malignant plasma cells. Moreover, this may provide an opportunity for an additional level of selectivity for tumor targeting.
Importantly, HM1.24-ETA′ not only induced apoptosis in plasmacytoma cell lines but was also an inductor of cell death in primary tumor samples from patients with plasma cell neoplasias. The somewhat higher concentrations of the immunotoxin required for maximal induction of apoptosis may be explained by the lower antigen density observed on freshly isolated tumor cells in comparison with MM cell lines. Alternatively, a reduced proliferation rate or alterations in protein synthesis may account for this phenomenon frequently observed with freshly isolated primary tumor cells.48 However, other cellular factors influencing toxin efficacy cannot be excluded. For example, upregulation of anti-apoptotic proteins such as Bcl2, BclXL or MCL-1 is frequently observed in MM49 and may render tumor cells more resistant to apoptosis-inducing agents such as immunotoxins. In addition, intracellular routing of the immunotoxin may be compromised by expression levels of the KDEL receptor or other proteins critical for a correct intracellular processing of the toxin.50
Recently, a phase II clinical trial in hairy cell leukemia with an immunotoxin similarly designed as HM1.24-ETA′ but directed against CD22 showed promising results, demonstrating that scFv-based ETA′ fusion proteins represent potent antitumor agents.23 Although the side effects still have to be considered, clinical studies with similarly designed molecules suggest that application of multiple cycles is feasible.51 At least in patients with B- and T-cell lymphoid malignancies, the formation of neutralizing antibodies was observed infrequently.51, 52 Recently, several optimized variants of truncated exotoxin A variants have been described to address this issue.53, 54 These novel variants may allow the design of immunotoxins with even less toxicity and immunogenicity.
CD317 was reported as an antigen with a highly restricted expression pattern, absent on many normal tissues.27 Interestingly, the CD317 antigen has been identified as an antiviral restriction factor that becomes upregulated upon type I IFN stimulation and limits the release of viral particles from infected cells.55 The IFN-induced upregulation of CD317 has also been demonstrated for tumor cells, suggesting a rationale for the combination of CD317-targeting agents with IFNs.29 A single report questions the CD317 expression pattern as being not restricted enough for suitable therapeutic targeting.31 The data presented here with human monocytes and HUVEC clearly demonstrate that surface expression of CD317 on healthy tissue does not inevitably result in killing of CD317-positive cells. Antigen surface expression density, the respective function in a given cellular background, the level of internalization and, as shown for monocytes, the activation status may govern susceptibility to HM1.24-ETA′ and other immunotoxins.39, 40, 41 A potent Fc-engineered CD317-specific IgG1 antibody was successfully tested in cynomolgus monkeys, demonstrating a significant and specific reduction of normal plasma cells in blood as well as bone marrow. Importantly, treatment was well tolerated and immunohistochemistry studies on normal tissues showed a similar staining pattern in humans and cynomolgus monkey.56 Furthermore, a native CD317 antibody has been tested in patients without adverse events, underlining that therapeutic administration of CD317-targeting agents is feasible.57
Interestingly, elevated surface expression levels of CD317 have also been reported on the tumor vasculature of B-cell lymphomas,58 on primary lung cancer cells29 and glioblastoma cells.28 Furthermore, gene expression profiling revealed elevated CD317 mRNA levels in endometrial cancer.30 These data indicate that CD317 may also represent a target structure for therapy in indications other than MM and that, in special situations, it may be possible to target both tumor and tumor vasculature.58
The HM1.24-ETA′ immunotoxin reported here represents an interesting immunotherapeutic option in patients with plasma cell disorders. Even in patients with a high tumor load, a suppressed immune system or limited hematopoiesis, including situations early after stem cell transplantation, such an approach can be used. In these settings, the action of immunotoxins in contrast to unconjugated or bispecific antibodies may be less dependent on functionally active immune effector cells. Furthermore, efficient responses to immunotoxin treatment most likely will not be influenced by FcR polymorphisms, as reported for unconjugated antibodies.8 Thus, the CD317 antigen may represent an interesting receptor for antigen-specific delivery of cytotoxic compounds, suggesting that HM1.24-ETA′ could serve as a potent immunoconjugate for treatment of plasma cell disorders and possibly other CD317-positive tumors.
References
Kyle RA, Rajkumar SV . Multiple myeloma. Blood 2008; 111: 2962–2972.
Anderson KC . The 39th David A. Karnofsky Lecture: bench-to-bedside translation of targeted therapies in multiple myeloma. J Clin Oncol 2012; 30: 445–452.
Ozaki S, Kosaka M, Wakatsuki S, Abe M, Koishihara Y, Matsumoto T . Immunotherapy of multiple myeloma with a monoclonal antibody directed against a plasma cell-specific antigen, HM1.24. Blood 1997; 90: 3179–3186.
Stein R, Qu Z, Cardillo TM, Chen S, Rosario A, Horak ID et al. Antiproliferative activity of a humanized anti-CD74 monoclonal antibody, hLL1, on B-cell malignancies. Blood 2004; 104: 3705–3711.
Tai YT, Dillon M, Song W, Leiba M, Li XF, Burger P et al. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood 2008; 112: 1329–1337.
Jakubowiak AJ, Benson DM, Bensinger W, Siegel DS, Zimmerman TM, Mohrbacher A et al. Phase I trial of anti-CS1 monoclonal antibody elotuzumab in combination with bortezomib in the treatment of relapsed/refractory multiple myeloma. J Clin Oncol 2012; 30: 1960–1965.
de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol 2011; 186: 1840–1848.
van de Winkel JG . Fc receptors: role in biology and antibody therapy. Immunol Lett 2010; 128: 4–5.
Goldmacher VS, Bourret LA, Levine BA, Rasmussen RA, Pourshadi M, Lambert JM et al. Anti-CD38-blocked ricin: an immunotoxin for the treatment of multiple myeloma. Blood 1994; 84: 3017–3025.
Huang YW, Richardson JA, Vitetta ES . Anti-CD54 (ICAM-1) has antitumor activity in SCID mice with human myeloma cells. Cancer Res 1995; 55: 610–616.
Ikeda H, Hideshima T, Fulciniti M, Lutz RJ, Yasui H, Okawa Y et al. The monoclonal antibody nBT062 conjugated to cytotoxic Maytansinoids has selective cytotoxicity against CD138-positive multiple myeloma cells in vitro and in vivo. Clin Cancer Res 2009; 15: 4028–4037.
Sapra P, Stein R, Pickett J, Qu Z, Govindan SV, Cardillo TM et al. Anti-CD74 antibody-doxorubicin conjugate, IMMU-110, in a human multiple myeloma xenograft and in monkeys. Clin Cancer Res 2005; 11: 5257–5264.
Tassone P, Goldmacher VS, Neri P, Gozzini A, Shammas MA, Whiteman KR et al. Cytotoxic activity of the maytansinoid immunoconjugate B-B4-DM1 against CD138+ multiple myeloma cells. Blood 2004; 104: 3688–3696.
Schrama D, Reisfeld RA, Becker JC . Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov 2006; 5: 147–159.
Wu AM, Senter PD . Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol 2005; 23: 1137–1146.
Linenberger ML . CD33-directed therapy with gemtuzumab ozogamicin in acute myeloid leukemia: progress in understanding cytotoxicity and potential mechanisms of drug resistance. Leukemia 2005; 19: 176–182.
Burris HA 3rd, Rugo HS, Vukelja SJ, Vogel CL, Borson RA, Limentani S et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol 2011; 29: 398–405.
Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med 2010; 363: 1812–1821.
Lutz RJ, Whiteman KR . Antibody-maytansinoid conjugates for the treatment of myeloma. MAbs 2009; 1: 548–551.
Azemar M, Schmidt M, Arlt F, Kennel P, Brandt B, Papadimitriou A et al. Recombinant antibody toxins specific for ErbB2 and EGF receptor inhibit the in vitro growth of human head and neck cancer cells and cause rapid tumor regression in vivo. Int J Cancer 2000; 86: 269–275.
Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ . Immunotoxin therapy of cancer. Nat Rev Cancer 2006; 6: 559–565.
Peipp M, Kupers H, Saul D, Schlierf B, Greil J, Zunino SJ et al. A recombinant CD7-specific single-chain immunotoxin is a potent inducer of apoptosis in acute leukemic T cells. Cancer Res 2002; 62: 2848–2855.
Kreitman RJ, Stetler-Stevenson M, Margulies I, Noel P, Fitzgerald DJ, Wilson WH et al. Phase II trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with hairy cell leukemia. J Clin Oncol 2009; 27: 2983–2990.
Kreitman RJ, Tallman MS, Robak T, Coutre S, Wilson WH, Stetler-Stevenson M et al. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol 2012; 30: 1822–1828.
Kellner C, Bleeker WK, Lammerts van Bueren JJ, Staudinger M, Klausz K, Derer S et al. Human kappa light chain targeted Pseudomonas exotoxin A--identifying human antibodies and Fab fragments with favorable characteristics for antibody-drug conjugate development. J Immunol Methods 2011; 371: 122–133.
Kupzig S, Korolchuk V, Rollason R, Sugden A, Wilde A, Banting G . Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic 2003; 4: 694–709.
Goto T, Kennel SJ, Abe M, Takishita M, Kosaka M, Solomon A et al. A novel membrane antigen selectively expressed on terminally differentiated human B cells. Blood 1994; 84: 1922–1930.
Wainwright DA, Balyasnikova IV, Han Y, Lesniak MS . The expression of BST2 in human and experimental mouse brain tumors. Exp Mol Pathol 2011; 91: 440–446.
Wang W, Nishioka Y, Ozaki S, Jalili A, Abe S, Kakiuchi S et al. HM1.24 (CD317) is a novel target against lung cancer for immunotherapy using anti-HM1.24 antibody. Cancer Immunol Immunother 2009; 58: 967–976.
Wong YF, Cheung TH, Lo KW, Yim SF, Siu NS, Chan SC et al. Identification of molecular markers and signaling pathway in endometrial cancer in Hong Kong Chinese women by genome-wide gene expression profiling. Oncogene 2007; 26: 1971–1982.
Erikson E, Adam T, Schmidt S, Lehmann-Koch J, Over B, Goffinet C et al. In vivo expression profile of the antiviral restriction factor and tumor-targeting antigen CD317/BST-2/HM1.24/tetherin in humans. Proc Natl Acad Sci USA 2011; 108: 13688–13693.
Burger R, Guenther A, Bakker F, Schmalzing M, Bernand S, Baum W et al. Gp130 and ras mediated signaling in human plasma cell line INA-6: a cytokine-regulated tumor model for plasmacytoma. Hematol J 2001; 2: 42–53.
Meister S, Schubert U, Neubert K, Herrmann K, Burger R, Gramatzki M et al. Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res 2007; 67: 1783–1792.
Peipp M, Ehlert C, Staudinger M, Kellner C, Burger R, Bruenke J et al. A recombinant bispecific antibody targeting HM1.24 for recruiting FcγRIII-positive cytotoxic effector cells against multiple myeloma. (submitted).
Barth S, Huhn M, Matthey B, Klimka A, Galinski EA, Engert A . Compatible-solute-supported periplasmic expression of functional recombinant proteins under stress conditions. Appl Environ Microbiol 2000; 66: 1572–1579.
Kellner C, Maurer T, Hallack D, Repp R, van de Winkel JG, Parren PW et al. Mimicking an induced self phenotype by coating lymphomas with the NKp30 ligand B7-H6 promotes NK cell cytotoxicity. J Immunol 2012; 189: 5037–5046.
Guenther A, Gordon S, Tiemann M, Burger R, Bakker F, Green JR et al. The bisphosphonate zoledronic acid has antimyeloma activity in vivo by inhibition of protein prenylation. Int J Cancer 2010; 126: 239–246.
Peipp M, Simon N, Loichinger A, Baum W, Mahr K, Zunino SJ et al. An improved procedure for the generation of recombinant single-chain Fv antibody fragments reacting with human CD13 on intact cells. J Immunol Methods 2001; 251: 161–176.
Masuyama N, Kuronita T, Tanaka R, Muto T, Hirota Y, Takigawa A et al. HM1.24 is internalized from lipid rafts by clathrin-mediated endocytosis through interaction with alpha-adaptin. J Biol Chem 2009; 284: 15927–15941.
Rollason R, Korolchuk V, Hamilton C, Jepson M, Banting G . A CD317/tetherin-RICH2 complex plays a critical role in the organization of the subapical actin cytoskeleton in polarized epithelial cells. J Cell Biol 2009; 184: 721–736.
Francisco JA, Kiener PA, Moran-Davis P, Ledbetter JA, Siegall CB . Cytokine activation sensitizes human monocytic and endothelial cells to the cytotoxic effects of an anti-CD40 immunotoxin. J Immunol 1996; 157: 1652–1658.
Nefedova Y, Landowski TH, Dalton WS . Bone marrow stromal-derived soluble factors and direct cell contact contribute to de novo drug resistance of myeloma cells by distinct mechanisms. Leukemia 2003; 17: 1175–1182.
Alderson RF, Kreitman RJ, Chen T, Yeung P, Herbst R, Fox JA et al. CAT-8015: a second-generation pseudomonas exotoxin A-based immunotherapy targeting CD22-expressing hematologic malignancies. Clin Cancer Res 2009; 15: 832–839.
Cao W, Bover L . Signaling and ligand interaction of ILT7: receptor-mediated regulatory mechanisms for plasmacytoid dendritic cells. Immunol Rev 2010; 234: 163–176.
Moffat JM, Segura E, Khoury G, Caminschi I, Cameron PU, Lewin SR et al. Targeting antigen to bone marrow stromal cell-2 expressed by conventional and plasmacytoid dendritic cells elicits efficient antigen presentation. Eur J Immunol 2013; 43: 595–605.
Yoo H, Park SH, Ye SK, Kim M . IFN-gamma-induced BST2 mediates monocyte adhesion to human endothelial cells. Cell Immunol 2011; 267: 23–29.
Rollason R, Korolchuk V, Hamilton C, Schu P, Banting G . Clathrin-mediated endocytosis of a lipid-raft-associated protein is mediated through a dual tyrosine motif. J Cell Sci 2007; 120 (Pt 21): 3850–3858.
Schwemmlein M, Peipp M, Barbin K, Saul D, Stockmeyer B, Repp R et al. A CD33-specific single-chain immunotoxin mediates potent apoptosis of cultured human myeloid leukaemia cells. Br J Haematol 2006; 133: 141–151.
Chauhan D, Hideshima T, Anderson KC . Apoptotic signaling in multiple myeloma: therapeutic implications. Int J Hematol 2003; 78: 114–120.
Smith DC, Spooner RA, Watson PD, Murray JL, Hodge TW, Amessou M et al. Internalized Pseudomonas exotoxin A can exploit multiple pathways to reach the endoplasmic reticulum. Traffic 2006; 7: 379–393.
Kreitman RJ, Wilson WH, Bergeron K, Raggio M, Stetler-Stevenson M, FitzGerald DJ et al. Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N Engl J Med 2001; 345: 241–247.
Kreitman RJ, Wilson WH, White JD, Stetler-Stevenson M, Jaffe ES, Giardina S et al. Phase I trial of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) in patients with hematologic malignancies. J Clin Oncol 2000; 18: 1622–1636.
Onda M, Beers R, Xiang L, Nagata S, Wang QC, Pastan I . An immunotoxin with greatly reduced immunogenicity by identification and removal of B cell epitopes. Proc Natl Acad Sci USA 2008; 105: 11311–11316.
Weldon JE, Xiang L, Chertov O, Margulies I, Kreitman RJ, FitzGerald DJ et al. A protease-resistant immunotoxin against CD22 with greatly increased activity against CLL and diminished animal toxicity. Blood 2009; 113: 3792–3800.
Liberatore RA, Bieniasz PD . Tetherin is a key effector of the antiretroviral activity of type I interferon in vitro and in vivo. Proc Natl Acad Sci USA 2011; 108: 18097–18101.
Tai YT, Horton HM, Kong SY, Pong E, Chen H, Cemerski S et al. Potent in vitro and in vivo activity of an Fc-engineered humanized anti-HM1.24 antibody against multiple myeloma via augmented effector function. Blood 2012; 119: 2074–2082.
Powles R, Yong K, Sirohi B . Humanized anti-HM1.24 antibody (AHM): phase I study in patients with relapsed or refractory myeloma. Japanese Multiple Myeloma Forum Proceedings, 3 November, 2003.
Schliemann C, Roesli C, Kamada H, Borgia B, Fugmann T, Klapper W et al. In vivo biotinylation of the vasculature in B-cell lymphoma identifies BST-2 as a target for antibody-based therapy. Blood 2010; 115: 736–744.
Acknowledgements
Heidi Bosse, Anja Muskulus and Britta von Below are kindly acknowledged for expert technical assistance. We thank Chugai Pharmaceuticals Co., Ltd for providing the humanized anti-HM1.24-IgG1 antibody used in some blocking experiments. This study was supported by a research grant from the Deutsche Forschungsgemeinschaft (DFG; PE 1425/2–1) to MP and RB, and a research grant from the Werner und Lara Kreitz Stiftung to MP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Blood Cancer Journal website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Staudinger, M., Glorius, P., Burger, R. et al. The novel immunotoxin HM1.24-ETA′ induces apoptosis in multiple myeloma cells. Blood Cancer Journal 4, e219 (2014). https://doi.org/10.1038/bcj.2014.38
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bcj.2014.38
This article is cited by
-
Spot the difference
Nature (2017)










