Abstract
To select an appropriate prognostic model in the treatment of mature T- and natural killer (NK) -cell lymphoma (peripheral T-cell lymphoma (PTCL) and NK-/T-cell lymphoma (NKTCL)) is crucial. This study investigated the usefulness of Ann Arbor staging classification International prognostic index (IPI), prognostic index for T-cell lymphoma (PIT) and International peripheral T-cell lymphoma Project score (IPTCLP). Between 2000 and 2009, 176 patients (122 males) with PTCL and NKTCL were diagnosed and treated from a single institute in Taiwan. The correlation between complete response (CR) rate, 3-year overall survival (OS), early mortality rate and four prognostic models was analyzed. Thirty-one patients received hematopoietic stem cell transplantation (HSCT) and were analyzed separately. Three-year OS rate was 34.7%, and anaplastic large-cell lymphoma harbored better outcome than others. IPI score had the lowest Akaike information criterion value (1081.197) and was the best score in predicting OS and early mortality (P=0.009). Ann Arbor stage classification can predict CR rate more precisely (P=0.006). OS was significantly better in patients who received HSCT, even in patients with unfavorable features compared with chemotherapy alone. All prognostic models were useful to evaluate the outcome of patients with PTCL and NKTCL but IPI score did best in predicting OS in PTCL and PIT score in NKTCL. This study also supported the role of HSCT in patients with high-risk or refractory PTCL or NKTCL.
Similar content being viewed by others
Introduction
Mature T- and natural killer (NK) -cell lymphomas, or the so-called peripheral T-cell lymphoma (PTCL) and NK-/T-cell lymphoma (NKTCL), are relatively rare, which account for 7–10% of non-Hodgkin’s lymphoma in the Western country1, 2, 3 and 20–30% in East Asia.4, 5, 6, 7, 8, 9 PTCL is a heterogeneous group of diseases and mostly presented with advanced stage and aggressive course, compared with B-cell lymphoid malignancy.10, 11, 12 Five-year overall survival (OS) rate was 30–49% in diffuse large B-cell lymphoma but only less than 30% in PTCL.2 Even so, some patients with PTCLs were cured by conventional chemotherapy.13, 14 The characteristics of patients with long-term survival or early mortality were not well defined. Even in patients with unfavorable prognostic features or refractory diseases, hematopoietic stem cell transplantation (HSCT) can prolong OS and disease-free survival (DFS).15, 16, 17, 18, 19 Therefore, the prognostic scores had a major role in discriminating patients who had good outcome or patients who needed intensive treatment.
Several prognostic models were designed to divide patients into low risk or high risk. Ann Arbor stage20 was applied to predict the prognosis and response to treatment in most lymphoma but some limitations existed. International prognostic index (IPI) score was used first in diffuse large B-cell lymphoma and the usefulness in PTCL was reported.12, 21 The prognostic index for T-cell lymphoma (PIT) score was designed for PTCL, not otherwise specified (PTCL-NOS) and based on age, performance status, lactate dehydrogenase (LDH) and bone marrow (BM) involvement.22 This score was further modified recently by replacing BM involvement by Ki-67 immunostain, a proliferation index.23 The usefulness of modified PIT needed to be tested in more studies.24 The fourth score was developed by the International peripheral T-cell lymphoma Project (IPTCLP) and included age performance status and platelet count.25
HSCT may have a role in the treatment of PTCL but the literatures in Asian population were limited. Thus, this study will focus on prognostic factors, comparison of prognostic models and the role of HSCT by analyzing the clinical features, laboratory data and outcomes of patients with PTCLs or NKTCLs from a single institute in Taiwan.
Patients and methods
Patients
After excluding age younger than 18 years, lymphoblastic lymphoma, primary cutaneous PTCL, mycosis fungoides, Sezary syndrome and primary cutaneous anaplastic large-cell lymphoma (ALCL), 176 patients were identified as PTCL or NKTCL by reviewing the database in the Department of Pathology and Laboratory Medicine, Taipei Veteran General Hospital between January 2000 and December 2009. The definite diagnoses were confirmed again by two well-experienced hematology pathologists according to the World Health Organization (WHO) classification.26 All patients were Chinese and can be assigned to one of the following subtypes: PTCL-NOS; extranodal NK-/T-cell lymphoma, nasal type (ENKL, nasal type); extranodal NK-/T-cell lymphoma (ENKL); anaplastic lymphoma kinase-positive (ALK-positive) ALCL; ALK-negative ALCL; angioimmunoblastic T-cell lymphoma (AITL); enteropathy-associated T-cell lymphoma (EATL); subcutaneous panniculitis-like T-cell lymphoma (SPTCL) or adult T-cell leukemia/lymphoma (ATLL). All subtypes of PTCLs and NKTCL were analyzed separately and together.
Baseline assessment and follow-up
All patients received baseline evaluation, standard treatment according to the guideline of lymphoma treatment in our institute and subsequent follow-up. Assessments included history taking, physical examination, laboratory tests, chest and abdominal computerized tomography scan and BM exam. Laboratory tests included complete blood count, liver and renal functions tests, electrolytes, LDH, immunoglobulin and β2-microglobulin levels. After completion of treatment, patients were recommended to receive follow-up every 3 months in first 3 years, every 6 months in the year 4–5 and annually then.
Treatment
All patients received treatment according guideline built in our institute. Most patients (n=126, 83.3%) received CHOP (cyclophosphamide, doxorubicin, vincristine and prednisolone)-based chemotherapy as induction therapy. If complete response (CR) was achieved in first follow-up, patients who were eligible for HSCT and without contraindications will receive HSCT. Second-line chemotherapy such as ESHAP (etoposide, cisplatin, cytarabine and methylprednisolone) or ICE (ifosphamide, carboplatin and etoposide) were administered if poor response to induction chemotherapy or relapsed/recurrent diseases. Radiotherapy alone or combined with chemotherapy was administrated to patients with ENKL, nasal type.
Criteria of response
According to Cheson’s criteria,27, 28 responses were classified as CR (total disappearance of all tumors), partial response (at least a 50% decrease in the sum of products of diameters of target lesions), stable disease (no objective decrease in tumor measurements qualifying as a partial response and no objective increase qualifying as progressive disease) or progressive disease (appearance of a new lesion(s) more than 1.5 cm in any axis, more than 50% increase in the sum of products of diameters of more than one node or more than 50% increase in the longest diameter of a previously identified node more than 1 cm in the short axis). The responses in our study were interpreted by radiologists based on computerized tomography scan or positron emission tomography. Rebiopsy was indicated in our patients if there were any unusual clinical presentations of tumors. OS was defined as the time from diagnosis to death due to any cause or to final follow-up. Early mortality was defined as patients who died within 3 months after diagnosis.
Prognostic score
Ann Arbor stage classification, IPI, PIT and IPTCLP were applied to predict OS, CR rate, 3-year OS and early mortality. Ann Arbor stage classification I was defined as low risk, stage II as low-intermediate risk, stage III as high-intermediate risk and stage IV as high risk.20
The IPI score was calculated by five parameters as age, ECOG (Eastern Cooperative Oncology Group) performance status (PS),29 LDH level (within normal limit versus above upper normal limit), extranodal involvement and Ann Arbor stage.21 The IPI score 0–1 was defined as low risk, 2 was low-intermediate risk, 3 was high-intermediate risk and 4–5 was high risk.
The PIT score was calculated by four parameters as age, ECOG PS, LDH level and BM involvement.22 Patients were classified into four risk groups as low risk (PIT score 0), low-intermediate risk (PIT score 1), high-intermediate risk (PIT score 2) and high risk (PIT score 3–4).
The IPTCLP score was calculated by three parameters as age, ECOG PS and thrombocytopenia.30 The IPTCLP score 0 was defined as low risk, 1 was low-intermediate risk, 2 was high-intermediate risk and 3 was high risk.
Statistical analysis
OS was defined as time from diagnosis to death from any causes or the date of last follow-up. Patients alive at last follow-up were censored. Factors associated with OS were analyzed univariately by Kaplan–Meier estimates31 and log-rank test. A Cox proportional hazards model was used for the multivariate analysis for survival.32 The discriminatory ability of each prognostic model was examined by using the Cox proportional hazards model, and the consequences of the Cox model were expressed with the Akaike information criterion (AIC), which reveals how the prognostic models affected OS.33, 34, 35 The lower the AIC, the more explanatory and informative the model is.36 In order to predict response (CR achievement) and early mortality (within 3 month of diagnosis), multivariate logistic regression analysis was carried out for comparisons of four prognostic models. A P-value less than 0.05 was considered statistically significant. SPSS version 18.0 (SPSS, Chicago, IL, USA) was used for all statistical analyses.
Results
Clinical characteristics and laboratory data
Of 176 patients, 81 patients (46.0%) had PTCL-NOS, 32 (18.2%) had ENKL, nasal type, 25 (14.2%) had AITL, 17 (9.7%) had ALK-negative ALCL, 6 (3.4%) had ALK-positive ALCL, 5 (2.8%) had ATLL, 5 (2.8%) had SPTCL, 3 (1.7%) had ENKL and 2 (1.1%) had EATL. Clinical characteristics of these subtypes were summarized in Table 1. The rarer subtypes, such as ATLL, SPTCL and EATL, were categorized as ‘others’. We analyzed ENKL, nasal type (n=32) and ENKL (n=3) together. There were male predominance in most subtypes, except ATLL and SPTCL. The median age was 62 years (range, 18–90 years) with AITL representing the oldest group of patients (median age, 75 years) and ALK-positive ALCL was the youngest group of patients (median age, 38.5 years; P=0.002). About 65.2% of the patients with ALCL and 17.1% of the patients with ENKL presented with an elevated LDH level (P=0.003). Advanced stage (Ann Arbor stage III or IV) was found more frequent in AITL patients (72.0%) than other subtypes, especially ENKL patients (42.9%). Patients with AITL had poorer initial ECOG PS (PS >1 in 52% of patients) than patients with other subtypes (P=0.01). Besides, patients with AITL had higher incidence of thrombocytopenia (48%) and hypoalbuminemia (64%, P=0.04).
Prognostic models
The relationship of subtypes and prognostic models were detailed in Table 2. All prognostic models were categorized into four risk groups, as low risk, low-intermediate risk, high-intermediate risk and high risk. Except ENKL and AITL groups, most subtypes had similar distribution in four risk stratifications. More than 92% of AITL patients had IPI score >1, 80% had PIT score >1 and 96% had IPTCLP score ⩾1. In contrast, 57.1% of ENKL patients had IPI score ⩽1, 65.7% had PIT score ⩽1 and 42.9% had IPTCLP score=0.
Treatment and outcome
The mean and median follow-up duration was 33.0 and 16.0 months, respectively. There were 150 patients (85.2%) who received induction chemotherapy, and 125 of them (83.3%) received CHOP or CHOP-like regimen (Table 3). Seven patients received radiotherapy as initial treatment due to contraindications to chemotherapy and nineteen patients did not received treatment because of poor general condition. CR rate after induction therapy was 30.6% in all patients, 32.9% in PTCL-NOS, 35.5% in ENKL, 13.6% in AITL and 36.8% in ALCL (33.3% in ALK-positive ALCL). There were no significant differences between most subtypes in CR rate, except AITL patients, with lowest CR rate. Early mortality rate was 22.7% in all patients, 22.9% in non-ALK-positive-ALCL PTCL patients, 25.9% in PTCL-NOS patients, 22.9% in ENKL patients, 24% in AITL patients and 17.4% in ALCL patients. Median OS in whole group was 15.75 months (range, 0.1–127 months). OS according to all subtypes was showed in Figure 1. Three-year OS rate in whole group was 34.7%, in non-ALK-positive-ALCL PTCL group was 33.5%, in PTCL-NOS group was 28.4%, in ENKL group was 37.1%, in AITL group was 32.0% and in ALCL group was 52.2% (in ALK-positive ALCL group was 66.7%).
Prognostic factors
We analyzed patients with ALCL separately from other PTCLs to avoid interference. On univariate analysis, age >60 years, fever as initial presentation, abnormal cytogenetic study from BM aspirate, mediastinal lymphadenopathy, extranodal involvement more than one site, ECOG PS >1, B symptoms, lymphopenia, hypoalbuminemia, elevated β2-microglobulin level, failure to achieve CR, advanced stage, IPI score >1, PIT score >1 and IPTCLP score ⩾1 can affect OS. Thus, we use these parameters into multivariate analysis. Finally, age >60 years, fever as initial presentation, BM involvement, thrombocytopenia, hypoalbuminemia and failure to achieve CR affected OS independently (Table 4).
In ALCL patients, age >60 years, PIT score >1, IPTCLP score ⩾1, hypogammaglobulinemia and failure to achieve CR were unfavorable variables affecting OS. In multivariate analysis, age >60 years (P=0.005, relative risk (RR)=8.05, 95% confidence interval (CI) 1.281–3.689), PIT score >1 (P=0.041, RR=4.16, 95% CI 1.023–3.126) and failure to achieve CR (P=0.019, RR=5.50, 95% CI 1.139–4.263) predicted OS independently. The difference in OS between ALK-positive and ALK-negative ALCLs was not significant statistically because of the limited case numbers.
Comparison of four prognostic models
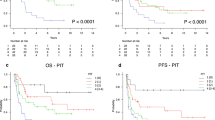
The comparisons of four prognostic models according to the CR rate, early mortality rate and 3-year OS rate in four risk stratifications were listed in Figure 2. Figure 2a showed that all prognostic models can predict CR achievement. In the final multivariate logistic regression model, Ann Arbor stage is the most important model predicting CR achievement (P=0.006). Figure 2b showed that all prognostic models predict early mortality. In the final multivariate logistic regression model, IPI score is the most important model predicting early mortality (P=0.009). Figure 2c showed four models and 3-year OS rate. AIC analysis in Ann Arbor stage were 1117.520, in IPI score was 1081.197, in PIT score was 1090.347 and in IPTCLP score was 1104.508. IPI score was better than other prognostic models in correlation with OS.
Outcome in patients receiving HSCT
Totally, 31 patients (18 males) received HSCT. Twenty-four patients received autologous HSCT (ASCT) and seven patients received allogeneic HSCT (Allo-SCT; three patients from sibling donor and four from unrelated donor). There were 16 patients with PTCL-NOS, 7 with ALCL, 4 with SPTCL, 2 with ENKL, 1 with ATLL and the last 1 with AITL. Before HSCT, 14 patients (45.2%) got CR after induction chemotherapy and HSCT was done for consolidation. Seventeen patients with refractory or relapsed diseases received HSCT as salvage treatment and 13 of them (76.4%) achieved CR after HSCT. The standard conditioning regimen of AHSCT in lymphoma was BEAC or BEAM (carmustine, etoposide, cytarabine, cyclophosphamide or melphalan). The standard conditioning regimen used in allogeneic HSCT was CyTBI (cyclophosphamide plus total body irradiation). After a median duration of follow-up of 64.8 months after HSCT, 22 of the 31 patients (71.0%) were alive and 19 patients (61.3%) were with disease-free status. Of the nine patients who died, four died from progressive disease, three died from treatment-related mortality (TRM; one from acute graft-versus-host disease and two from sepsis), one died from pneumonia 36 months after HSCT and one died from secondary malignancy. The TRM rate was 9.7%. One patient was diagnosed as PTCL-NOS and received ASCT in 2003. She suffered from T-cell acute lymphoblastic leukemia/lymphoma in 2008 and received Allo-SCT. She was still disease-free now. The actuarial 3-year OS was 80.6% and median OS was 71 months (range, 7.2–127). After univariate analysis of predictive factors, only extranodal involvement more than one site affected OS significantly (P=0.005). The differences between patients receiving HSCT or not were listed in Table 5. The patients who received HSCT were with younger age, better ECOG PS and had higher CR rate before HSCT than patients who received conventional therapy only.
Discussion
PTCLs and NKTCLs were more prevalent in Eastern Asia and the distribution of subtypes, prognosis and outcome were different from other ethnic populations. There were more ENKLs (19.9%) and less ALCLs (14.2%) in our cohort than in western country, and this geographic variation was also observed by other studies.4, 9, 13, 37 Almost all PTCLs and NKTCLs were aggressive and associated with poor prognosis, except ALK-positive ALCL. ALK-status influenced the treatment response and outcome significantly.38 Besides, several studies were reported to identify prognostic factors in patients with PTCL, such as Ki-67, absolute lymphocyte count, pretreatment serum total protein, IPI score and PIT score.12, 23, 39, 40, 41, 42, 43, 44, 45 Initial multivariate analysis in all PTCLs and NKTCLs showed that ALCL subtype and age more than 60 years were most important variables. Thus, we analyzed patients with ALCL separately. There were only six patients with ALK-positive ALCL in our study therefore, we combined ALK-positive and ALK-negative ALCL for analysis. The difference of outcome between ALK-positive and -negative ALCL was not significant statistically in our patient cohort.
In patients with non-ALCL PTCL, age >60 years, fever, BM involvement, elevated LDH level, thrombocytopenia, hypoalbuminemia and failure to achieve CR affected the outcome independently. In patients with ALCL, age >60 years, PIT >1 and failure to achieve CR were independent negative prognostic factors.
In comparison with aggressive B-cell lymphoma, the result of conventional chemotherapy was poor. CHOP remained the standard chemotherapy. A recent study showed that etoposide combined with CHOP may improve the outcome in young patients with PTCL.46 Alemtuzumab, an anti-CD 52 monoclonal antibody, was combined with CHOP as induction chemotherapy in patients with PTCL. Gallamini et al.47 reported a wonderful result that the CR rate achieved 70% although some manageable infections. But infection might limit the use of this agent. In salvage setting, pralatrexate, a novel antifolate agent, was approved as single agent in relapsed PTCL by the US Food and Drug Administration.48 The complete and partial response rates in 115 patients with relapsed or refractory PTCL were 10% and 17%, respectively.49, 50 ASCT or Allo-SCT had a role in salvage treatment or consolidation treatment for high-risk patients. Several prognostic models, including Ann Arbor stage classification, IPI score, PIT score and IPTCLP score, demonstrated their usefulness to identify high-risk patients. Gutiérrez-García et al.24 compared IPI, PIT, IPTCLP and modified PIT scores by CR rate, early mortality, 5-year DFS and 5-year OS. They concluded that IPTCLP score was the best score for OS, and IPI score had the best ability to predict CR. In Asia, similar studies were absent. We use logistic regression for analyses of CR rate and early mortality and AIC for correlation with OS. The AIC analysis, which is based on the Cox proportional hazards model, represents an overall assessment of the prognostic system and is the most important reference for the comparison across different staging systems. Thus, AIC was used for analysis of OS. In our study, Ann Arbor stage correlated with CR rate best (P=0.006). This may be because larger tumor burden lowered the CR rate. IPI score, however, predicted early mortality and 3-year OS best.
In previous studies, intensive treatment, such as ASCT or Allo-SCT, showed improvements in OS and DFS as salvage treatment or consolidation therapy in high-risk patients. Despite inferior response to conventional chemotherapy, patients with PTCL did not have inferior outcome after ASCT in two studies.51, 52 In a salvage setting, the 3-year OS after ASCT was approximately 36–48%.53, 54, 55, 56, 57 In comparison with second-line chemotherapy, ASCT improved OS and DFS. There were several prospective trials about the role of frontline ASCT as consolidation therapy in high-risk patients.19, 58, 59, 60 The TRM was about 3–4.8% and 3-year OS rate ranged from 50 to 75%. The 12-year OS rate was 34% in one study by Corradini et al.58 Frontline ASCT appeared to be an effective approach for the consolidation in high-risk PTCL. Age-adjusted IPI score and β2-microglobulin can predict the outcome.54 Some PTCLs relapsed even after ASCT and Allo-SCT was tried in some patients. The rationale was the graft-versus-lymphoma effect.17, 61 But the data of Allo-SCT were limited in relapsed or high-risk PTCL with poor outcome, such as AITL, SPTCL or hepatosplenic γδ T-cell lymphoma.17, 61, 62, 63, 64, 65 Unfortunately, most patients with AITL were older than 70 years and cannot tolerate intensive treatment.66 Because of the benefits of HSCT overweighed the acceptable TRM. We analyzed 31 patients receiving HSCT. Twenty-four and seven patients received ASCT and Allo-SCT, respectively. Fourteen patients (45.2%, thirteen ASCT and one Allo-SCT) were in CR status before transplantation. The TRM was 9.7%, 3-year OS rate was 80.6% and median OS was 71 months. The outcomes of patients receiving ASCT or Allo-SCT were even better than patients with low-risk IPI score, no matter in salvage setting or in consolidation setting (Figure 3). The negative predictive factor in patients receiving HSCT was extranodal involvement more than one site. Ann Arbor stage, IPI, PIT neither IPTCLP scores did not affect the outcome of HSCT. Owing to the advances in ASCT or reduced-intensity Allo-SCT, patients with high-risk PTCL may benefit from these novel treatments without much toxicity or TRM.
There were some limitations in our study, the retrospective design caused selection bias in interpretation of the data of HSCT. Besides, the sample size in several subtypes was too small to be analyzed separately. The NKTCL prognostic score was reported by Lee et al.67 including LDH, B symptoms, lymph nodes, N1-N3 involvement and advanced Ann Arbor stage. In our cohort, independent prognostic factors in patients with ENKL included age >60 years, ECOG PS >1, advanced stage, extranodal involvement >1 site, IPI score >1 and lymphopenia. By analyzing five scores (stage, IPI, PIT, IPTCLP and NKTCL scores) in our patients with NKTCL, PIT score (AIC: 120.726) was better than the other four scores in predicting OS. But the case number was too small to be analyzed without bias. (Supplementary Figure and Table).
In conclusion, IPI score correlated with the OS in patients with PTCLs better than other three prognostic models. In NKTCL, PIT score was the ideal score in evaluating OS. Besides, Ann Arbor stage can predict the CR rate. Our study supported the use of HSCT in selected patients with PTCLs or NKTCLs, but prospective randomized trial are needed to confirm the benefit of frontline HSCT in high-risk patients responding to chemotherapy.
References
Ascani S, Zinzani PL, Gherlinzoni F, Sabattini E, Briskomatis A, de Vivo A et al. Peripheral T-cell lymphomas. Clinico-pathologic study of 168 cases diagnosed according to the R.E.A.L. classification. Ann Oncol 1997; 8: 583–592.
The Non-Hodgkin′s Lymphoma Classification Project. A clinical evaluation of the International Lymphoma Study Group Classification of non-Hodgkin′s lymphoma. Blood 1997; 89: 3909–3918.
Harris N, Jaffe E, Stein H, Banks P, Chan J, Cleary M et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group [see comments]. Blood 1994; 84: 1361–1392.
Yoon SO, Suh C, Lee DH, Chi H-S, Park CJ, Jang S-S et al. Distribution of lymphoid neoplasms in the Republic of Korea: Analysis of 5318 cases according to the World Health Organization classification. Am J Hematol 2010; 85: 760–764.
Chen W-L, Tsai W-C, Chao T-Y, Sheu L-F, Chou J-M, Kao W-Y et al. The clinicopathological analysis of 303 cases with malignant lymphoma classified according to the World Health Organization classification system in a single institute of Taiwan. Ann Hematol 2010; 89: 555–562.
Kadin ME, Berard CW, Nanba K, Wakasa H . Lymphoproliferative diseases in Japan and Western countries: proceedings of the United States-Japan seminar, September 6 and 7, 1982, in Seattle, Washington. Hum Pathol 1983; 14: 745–772.
Anderson JR, Armitage JO, Weisenburger DD . For the Non-Hodgkin′s Lymphoma Classification Project. Epidemiology of the non-Hodgkin′s lymphomas: distributions of the major subtypes differ by geographic locations. Ann Oncol 1998; 9: 717–720.
Ameen R, Sajnani K, Albassami A, Refaat S . Frequencies of non-Hodgkin′s lymphoma subtypes in Kuwait: comparisons between different ethnic groups. Ann Hematol 2010; 89: 179–184.
Ko Y-H, Kim C-W, Park C-S, Jang H-K, Lee S-S, Kim S-H et al. REAL classification of malignant lymphomas in the republic of korea. Cancer 1998; 83: 806–812.
Tomita N, Motomura S, Hyo R, Takasaki H, Takemura S, Taguchi J et al. Comparison of peripheral T-cell lymphomas and diffuse large B-cell lymphoma. Cancer 2007; 109: 1146–1151.
Coiffier B, Brousse N, Peuchmaur M, Berger F, Gisselbrecht C, Bryon PA et al. Peripheral T-cell lymphomas have a worse prognosis than B-cell lymphomas: A prospective study of 361 immunophenotyped patients treated with the LNH-84 regimen. Ann Oncol 1990; 1: 45–50.
Gisselbrecht C, Gaulard P, Lepage E, Coiffier B, Briere J, Haioun C et al. Prognostic significance of T-cell phenotype in aggressive non-Hodgkin′s lymphomas. Blood 1998; 92: 76–82.
International T-Cell Lymphoma Project. International peripheral T-Cell and natural killer/T-Cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol 2008; 26: 4124–4130.
Rüdiger T, Weisenburger DD, Anderson JR, Armitage JO, Diebold J, MacLennan KA et al. Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): results from the non-Hodgkin′s lymphoma classification project. Ann Oncol 2002; 13: 140–149.
Rodríguez J, Conde E, Gutiérrez A, Arranz R, León Á, Marín J et al. The results of consolidation with autologous stem-cell transplantation in patients with peripheral T-cell lymphoma (PTCL) in first complete remission: the Spanish Lymphoma and Autologous Transplantation Group experience. Ann Oncol 2007; 18: 652–657.
Chen AI, McMillan A, Negrin RS, Horning SJ, Laport GG . Long-term results of autologous hematopoietic cell transplantation for peripheral T cell lymphoma: the Stanford experience. Biol Blood Marrow Transplant 2008; 14: 741–747.
Le Gouill S, Milpied N, Buzyn A, Peffault De Latour R, Vernant J-P, Mohty M et al. Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. J Clin Oncol 2008; 26: 2264–2271.
Numata A, Miyamoto T, Ohno Y, Kamimura T, Kamezaki K, Tanimoto T et al. Long-term outcomes of autologous PBSCT for peripheral T-cell lymphoma: retrospective analysis of the experience of the Fukuoka BMT group. Bone Marrow Transplant 2010; 45: 311–316.
Reimer P, Rudiger T, Geissinger E, Weissinger F, Nerl C, Schmitz N et al. Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol 2009; 27: 106–113.
Lister T, Crowther D, Sutcliffe S, Glatstein E, Canellos G, Young R et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin′s disease: Cotswolds meeting (published erratum appears in J Clin Oncol 1990; 8(9): 1602). J Clin Oncol 1989; 7: 1630–1636.
The International Non-Hodgkin′s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin′s lymphoma. N Engl J Med 1993; 329: 987–994.
Gallamini A, Stelitano C, Calvi R, Bellei M, Mattei D, Vitolo U et al. Peripheral T-cell lymphoma unspecified (PTCL-U): a new prognostic model from a retrospective multicentric clinical study. Blood 2004; 103: 2474–2479.
Went P, Agostinelli C, Gallamini A, Piccaluga PP, Ascani S, Sabattini E et al. Marker expression in peripheral T-cell lymphoma: a proposed clinical-pathologic prognostic score. J Clin Oncol 2006; 24: 2472–2479.
Gutiérrez-García G, García-Herrera A, Cardesa T, Martínez A, Villamor N, Ghita G et al. Comparison of four prognostic scores in peripheral T-cell lymphoma. Ann Oncol 2010.
Project IT-CL. International Peripheral T-Cell and Natural Killer/T-Cell Lymphoma Study. Pathology findings and clinical outcomes. J Clin Oncol 2008; 26: 4124–4130.
Swerdlow SH, Campo E, Harris NL et al (eds). WHO classification of tumours, WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th edn, vol. 2 IARC: Lyon, 2008.
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM et al. Report of an international workshop to standardize response criteria for non-Hodgkin′s lymphomas. J Clin Oncol 1999; 17: 1244.
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25: 579–586.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5: 649–656.
Vose JM . The International PTCL Project. International peripheral T-Cell lymphoma (PTCL) clinical and pathologic review project. Poor outcome by prognostic indices and lack of efficacy with anthracyclines. ASH Annu Meet Abstr 2005; 106: 811.
Meier ELKaP. Non parametric estimation for incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Mantel N . Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 1966; 50: 163–170.
AR F. Clinical biostatistics. XVI. The process of prognostic stratification. Clin Pharmacol Ther 1972; 13: 609–624.
Hosmer DW, Hosmer T, Le Cessie S, Lemeshow S . A comparison of goodness-of-fit tests for the logistic regression model. Stat Med 1997; 16: 965–980.
Hsu C-Y, Hsia C-Y, Huang Y-H, Su C-W, Lin H-C, Lee P-C et al. Selecting an optimal staging system for hepatocellular carcinoma. Cancer 2010; 116: 3006–3014.
Forster MR . Key concepts in model selection: performance and generalizability. J Math Psychol 2000; 44: 205–231.
Kobayashi R, Yamato K, Tanaka F, Takashima Y, Inada H, Kikuchi A et al. Retrospective analysis of non-anaplastic peripheral T-cell lymphoma in pediatric patients in Japan. Pediatr Blood Cancer 2010; 54: 212–215.
Gascoyne RD, Aoun P, Wu D, Chhanabhai M, Skinnider BF, Greiner TC et al. Prognostic significance of anaplastic lymphoma kinase (ALK) protein expression in adults with anaplastic large cell lymphoma. Blood 1999; 93: 3913–3921.
Piva R, Agnelli L, Pellegrino E, Todoerti K, Grosso V, Tamagno I et al. Gene expression profiling uncovers molecular classifiers for the recognition of anaplastic large-cell lymphoma within peripheral T-cell neoplasms. J Clin Oncol 2010; 28: 1583–1590.
Agostinelli C, Piccaluga PP, Went P, Rossi M, Gazzola A, Righi S et al. Peripheral T cell lymphoma, not otherwise specified: the stuff of genes, dreams and therapies. J Clin Pathol 2008; 61: 1160–1167.
Castillo JJ, Morales D, Quinones P, Cotrina E, Desposorio C, Beltran B . Lymphopenia as a prognostic factor in patients with peripheral T-cell lymphoma, unspecified. Leuk Lymphoma 2010; 51: 1822–1828.
Huang JJ, Jiang WQ, Lin TY, Huang Y, Xu RH, Huang HQ et al. Absolute lymphocyte count is a novel prognostic indicator in extranodal natural killer/T-cell lymphoma, nasal type. Ann Oncol 2011; 22: 149–155.
López-Guillermo A, Cid J, Salar A, López A, Montalbán C, Castrillo JM et al. Peripheral T-cell lymphomas: Initial features, natural history, and prognostic factors in a series of 174 patients diagnosed according to the R.E.A.L. Classification. Ann Oncol 1998; 9: 849–855.
Piccaluga P, Agostinelli C, Gazzola A, Mannu C, Bacci F, Sabattini E et al. Prognostic markers in peripheral T-cell lymphoma. Curr Hematol Malig Rep 2010; 5: 222–228.
Watanabe T, Kinoshita T, Itoh K, Yoshimura K, Ogura M, Kagami Y et al. Pretreatment total serum protein is a significant prognostic factor for the outcome of patients with peripheral T/natural killer-cell lymphomas. Leuk Lymphoma 2010; 51: 813–821.
Schmitz N, Trumper L, Ziepert M, Nickelsen M, Ho AD, Metzner B et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood 2010; 116: 3418–3425.
Gallamini A, Zaja F, Patti C, Billio A, Specchia MR, Tucci A et al. Alemtuzumab (Campath-1H) and CHOP chemotherapy as first-line treatment of peripheral T-cell lymphoma: results of a GITIL (Gruppo Italiano Terapie Innovative nei Linfomi) prospective multicenter trial. Blood 2007; 110: 2316–2323.
Thompson CA . FDA approves pralatrexate for treatment of rare lymphoma. Am J Health Syst Pharm 2009; 66: 1890.
O′Connor OA, Horwitz S, Hamlin P, Portlock C, Moskowitz CH, Sarasohn D et al. Phase II-I-II study of two different doses and schedules of pralatrexate, a high-affinity substrate for the reduced folate carrier, in patients with relapsed or refractory lymphoma reveals marked activity in T-cell malignancies. J Clin Oncol 2009; 27: 4357–4364.
Toner LE, Vrhovac R, Smith EA, Gardner J, Heaney M, Gonen M et al. The schedule-dependent effects of the novel antifolate pralatrexate and gemcitabine are superior to methotrexate and cytarabine in models of human non-Hodgkin′s lymphoma. Clin Cancer Res 2006; 12: 924–932.
Vose J, Peterson C, Bierman P, Weisenburger D, Linder J, Harrington D et al. Comparison of high-dose therapy and autologous bone marrow transplantation for T-cell and B-cell non-Hodgkin′s lymphomas. Blood 1990; 76: 424–431.
Sohn BS, Park I, Kim EK, Yoon DH, Lee SS, Kang BW et al. Comparison of clinical outcome after autologous stem cell transplantation between patients with peripheral T-cell lymphomas and diffuse large B-cell lymphoma. Bone Marrow Transplant 2009; 44: 287–293.
Kewalramani T, Zelenetz AD, Teruya-Feldstein J, Hamlin P, Yahalom J, Horwitz S et al. Autologous transplantation for relapsed or primary refractory peripheral T-cell lymphoma. Br J Haematol 2006; 134: 202–207.
Rodriguez J, Conde E, Gutierrez A, Lahuerta JJ, Arranz R, Sureda A . The adjusted International Prognostic Index and beta-2-microglobulin predict the outcome after autologous stem cell transplantation in relapsing/refractory peripheral T-cell lymphoma. Haematologica 2007; 92: 1067–1074.
Feyler S, Prince HM, Pearce R, Towlson K, Nivison-Smith I, Schey S . The role of high-dose therapy and stem cell rescue in the management of T-cell malignant lymphomas: a BSBMT and ABMTRR study. Bone Marrow Transplant 2007; 40: 443–450.
Rodriguez J, Munsell M, Yazji S, Hagemeister FB, Younes A, Andersson B et al. Impact of high-dose chemotherapy on peripheral T-cell lymphomas. J Clin Oncol 2001; 19: 3766–3770.
Jantunen E, Wiklund T, Juvonen E, Putkonen M, Lehtinen T, Kuittinen O . Autologous stem cell transplantation in adult patients with peripheral T-cell lymphoma: a nation-wide survey. Bone Marrow Transplant 2004; 33: 405–410.
Corradini P, Tarella C, Zallio F, Dodero A, Zanni M, Valagussa P . Long-term follow-up of patients with peripheral T-cell lymphomas treated up-front with high-dose chemotherapy followed by autologous stem cell transplantation. Leukemia 2006; 20: 1533–1538.
Rodriguez J, Conde E, Gutierrez A, Arranz R, Leon A, Marin J . Frontline autologous stem cell transplantation in high-risk peripheral T-cell lymphoma: a prospective study from the Gel-Tamo Study Group. Eur J Haematol 2007; 79: 32–38.
Mercadal S, Briones J, Xicoy B, Pedro C, Escoda L, Estany C . Intensive chemotherapy (high-dose CHOP/ESHAP regimen) followed by autologous stem-cell transplantation in previously untreated patients with peripheral T-cell lymphoma. Ann Oncol 2008; 19: 958–963.
Corradini P, Dodero A, Zallio F, Caracciolo D, Casini M, Bregni M . Graft-versus-lymphoma effect in relapsed peripheral T-cell non-Hodgkin′s lymphomas after reduced-intensity conditioning followed by allogeneic transplantation of hematopoietic cells. J Clin Oncol 2004; 22: 2172–2176.
Wulf GG, Hasenkamp J, Jung W, Chapuy B, Truemper L, Glass B . Reduced intensity conditioning and allogeneic stem cell transplantation after salvage therapy integrating alemtuzumab for patients with relapsed peripheral T-cell non-Hodgkin′s lymphoma. Bone Marrow Transplant 2005; 36: 271–273.
Tanosaki R, Kim S, Yamazaki S, Fukuda T, Mori S, Heike Y . A retrospective single institute analysis of 127 lymphoma patients who underwent allogeneic stem cell transplantation: impact on peripheral T-cell lymphoma (PTCL) including ATLL. Blood 2006; 108: 3031.
Savage HM OL, Toze KJ, Connors CL, Gascoyne JM, Mourad RD . YA. Allogeneic stem cell transplantation as treatment for relapsed and high-risk peripheral T-Cell lymphoma. Blood 2007; 110: 3040.
Kyriakou C, Canals C, Finke J, Kobbe G, Harousseau J-L, Kolb H-J et al. Allogeneic stem cell transplantation is able to induce long-term remissions in angioimmunoblastic T-cell lymphoma: a retrospective study from the Lymphoma Working Party of the European Group for blood and marrow transplantation. J Clin Oncol 2009; 27: 3951–3958.
Lin H-N, Liu C-Y, Hong Y-C, Pai J-T, Yang C-F, Yu Y-B et al. Clinical features and prognostic factors of angioimmunoblastic T-cell lymphoma in Taiwan: a single-institution experience. Leuk Lymphoma 2010; 51: 2208–2214.
Lee J, Suh C, Park YH, Ko YH, Bang SM, Lee JH et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol 2006; 24: 612–618.
Acknowledgements
This study was supported by the Clinical Oncology Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Blood Cancer Journal website
Supplementary information
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Lin, HN., Liu, CY., Pai, JT. et al. How to predict the outcome in mature T and NK cell lymphoma by currently used prognostic models?. Blood Cancer Journal 2, e93 (2012). https://doi.org/10.1038/bcj.2012.23
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bcj.2012.23
Keywords
This article is cited by
-
Dysregulated choline metabolism in T-cell lymphoma: role of choline kinase-α and therapeutic targeting
Blood Cancer Journal (2015)
-
Analysis of prognostic factors and comparison of prognostic scores in peripheral T cell lymphoma, not otherwise specified: a single-institution study of 105 Chinese patients
Annals of Hematology (2015)