Abstract
Tumor cells produce and secrete more nucleic acids, proteins and lipids than normal cells. These molecules are transported in the blood or around the cells in membrane-encapsulated exosomes. Tumor-derived or tumor-associated exosomes (usually 30–100 nm in diameter) contain abundant biological contents resembling those of the parent cells along with signaling messengers for intercellular communication involved in the pathogenesis, development, progression, and metastasis of cancer. As these exosomes can be detected and isolated from various body fluids, they have become attractive new biomarkers for the diagnosis and prognosis of cancer. Furthermore, tumor exosomes have also attracted increasing attention due to their potential as novel therapeutic strategies for the treatment of cancers. On the one hand, the lipid bilayer membrane-encapsulated vesicles are promising carriers of drugs and other therapeutic materials targeting specific cancer cells. On the other hand, tumor exosomes are important mediators for modulation of the microenvironment that orchestrates events critical to the growth and metastasis of cancer cells as well as chemoresistance. Here, we summarize the advances in our understanding of tumor-associated or tumor-derived exosomes in recent years, and discuss their roles in cancer development, progression, invasion, and metastasis of cancers and, more importantly, their potential in strategies for precision therapy of various cancers as well as important caveats.
Similar content being viewed by others
Introduction
Tumor cells, similar to normal cells, are factories that constantly produce and export various biological molecules. These molecules are released from the cells and transported in the blood or around the cells in unique nano-sized lipid bilayer membrane vesicles1. A group of these membrane vesicles with a diameter of approximately 40 nanometer (nm) were initially identified from two types of cancer cells with electron microscopy by Trams et al in 19812. Later, Johnstone et al reported vesicle externalization from multivesicular bodies using immunoelectron microscopy during reticulocyte maturation, and then, they defined the exosomes with a diameter of 30–100 nm as microvesicles3. At this very early stage, exosomes were erroneously identified as cellular garbage until the emergence of dendritic cell (DC)-derived exosomes that contributed to the inhibition of cancer cell growth by increasing the proliferation of cytotoxic T lymphocytes4. Indeed, these pioneer studies promoted the application of exosomes not only in cancer research but also in the general field of cell biology, physiology, and pharmacology. In 2013, the Nobel Prize in Physiology or Medicine was awarded to three scientists “for their discoveries of machinery regulating vesicle traffic, a major transport system in our cells”5. Randy SCHEKMAN discovered a set of genes that were required for vesicle traffic6. James ROTHMAN identified protein machinery that allows vesicles to fuse with their targets to permit vesicle transfer7. Thomas SÜDHOF revealed how signals instruct the cell to organize microvesicle exosomes and deliver them to the right place at the right time8. Indeed, later studies consistently found that disturbances in the exquisitely precise control system for the transport and delivery of microvesicles/exosomes have deleterious effects and contribute to many pathological conditions, such as immunological disorders9,10 and cancers11,12. More details about the general physiology, pathology, functionality, and roles in cancer metastasis and therapy of these molecules can be found in several recent excellent review articles13,14,15,16,17. To date, tumor-associated or tumor-derived exosomes have been shown to be extensively involved in the intercellular substance exchange and signal communication between the tumor and the host and play crucial roles in the pathogenesis, development, invasion, and metastasis of cancer cells; more importantly, their potential as novel therapeutic strategies for precision therapy of various cancers has been demonstrated. In this review, we summarize the advances in our understanding of tumor exosomes in recent years and discuss their roles in the development, invasion, and metastasis of cancer cells, and more importantly, the potential and limitations for the development of tumor exosome-based therapeutic strategies for cancer treatment.
Characteristics of tumor-derived and tumor-associated exosomes
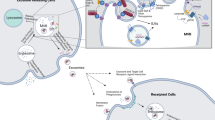
Tumor cells continuously secrete membrane vesicles to the extracellular environment. These tumor-derived exosomes represent novel vehicles for cell-cell communication networks and new components of a supportive microenvironment for localization, proliferation, and survival of tumor cells2,18,19,20,21. Trams et al first used the term “exosomes” to describe membrane-enclosed structures of variable sizes budding from the cell membranes of C-6 glioma or N-18 neuroblastoma cells2. Exosomes are now often used to describe small extracellular membrane vesicles with a diameter of 30–150 nm generated via an endocytic pathway. Exosomes released by malignant tumor cells contain specific repertoires of molecules, including proteins, lipids, DNA molecules, miRNAs, mRNAs and non-coding RNAs, which are important for cancer cell communication with the environment. In the ExoCarta database, 286 studies focused on exosomes were found together with the contents in the exosomes, which included 41860 proteins, 7540 RNA molecules and 1116 lipids22,23,24. Specific sorting mechanisms have been proposed for controlling the sorting and loading of selected molecules into exosomes25.
In addition to cancer cells, multiple other cell types, including adult stem cells, stromal cells, and cancer stem cells, are known to communicate with each other through their exosomes within the tumor microenvironment26,27,28,29,30,31,32. Accumulating evidence has demonstrated that these tumor-associated exosomes mediate the interactions among different types of cells within the tumor microenvironment, thereby modulating tumor development, progression, and metastasis29,30,31,32. These studies have also provided new insight into how these cells interact with each other to promote resistance to anti-cancer drug therapy and contribute to tumor recurrence through exosome signaling33,34,35,36,37. While the detailed cellular and molecular mechanisms for the interaction between cancer cells and their surrounding cells, and thereby tumor progression and metastasis, remain to be further elucidated, current evidence has demonstrated that extracellular microvesicles, especially exosomes, are transferred among cancer cells and their surrounding cancer stem cells, adult stem cells and stromal cells, providing another layer of complexity in cancer cell biology associated with the tumor microenvironment and tumor progression.
Although there are differences in the chemical composition and biological functions of exosomes in various tissues and cells, the process of exosome formation is similar. Initially, primary endosomes are formed after entrapment of the cytoplasmic membrane, and then, the endosomes may fuse with the lysosomes, which trigger the formation of vesicles in the lumen after releasing the cellular contents into the endosomes. Then, these primary endosomes are shifted to secondary endosomes and are called multivesicular bodies38. Next, the multivesicular bodies fuse with a specific part of the cellular membrane. The multivesicular bodies fuse with the lysosomes, followed by fusing with the cellular membrane and secretion of vesicles through exocytosis via regulation of the GTPase and NsF coupling protein receptor. These substances were reported to be detected in blood, lymph fluid, urine, saliva, breast milk and ascites39.
Upon uptake of exosomes by the target cells, the exosome content may affect the biological behaviors and induce phenotypic changes in target recipient cells by several mechanisms. Exosome miRNAs, for example, can silence mRNA targets and influence cellular functions40,41,42. Exosomes released by acute myeloid leukemia cells affected the proliferation and migration of bone marrow stromal cells43, and multiple myeloma exosomes enhanced angiogenesis44.
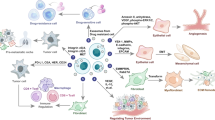
Tumor exosomes in invasion and metastasis of cancer cells
Not all cancer cells, or at least cancer cells in the initial early stages, show an invasive capacity. Invasion gradually progresses during the pathogenesis and development of most cancer cells. The biological and genetic alterations of the daughter cells of cancer cells may be transferred through exosomes after multiple rounds of division and proliferation, which then result in the sharing of biological features among cancer cells. Metastasis is a complex multistep process that involves cancer cell invasion, survival in blood vessels, and attachment to and colonization of distant host organs. A variety of molecular and functional mediators in cell interactions are involved in cancer metastasis. Tumor exosomes are now known to influence almost every step of the signaling cascade of cancer cell metastasis and thus can be targeted as a therapeutic approach. Al-Nedawi et al studied the effects of intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from glioma cells with EGFR mutations45. Abundant expression of anti-apoptotic genes was observed in the recipient cells, together with increased non-adherent growth of cancer cells and invasion. Similarly, transfer of DKO-1 (mutant KRAS allele only) to DKs-8 (wild-type KRAS allele only) resulted in enhanced three-dimensional growth of the wild-type KRAS-expressing non-transformed cells as exosomes from mutant KRAS cells contained many tumor-promoting proteins, including KRAS, EGFR, SRC family kinases and integrins46. These findings indicated that exosomes may serve as an important signal transfer vehicle and a vector for the delivery of genetic information47. Le et al indicated that in murine cancer and human xenograft models, extracellular vesicles from highly metastatic breast cancer cells promoted metastasis of otherwise weakly metastatic cells either nearby or at distant sites and induced these cells to colonize distant tissues in a miR-200-dependent manner48. These results demonstrated that uptake of extracellular vesicles contributed to the transfer of metastatic capability in breast cancer cells.
It has been well established that organs of future cancer metastasis are not passive receivers of circulating cancer cells. The tumor microenvironment is closely related to cancer metastasis. Before metastasis, cancer cells may modulate the microenvironment of the target organs through exosomes to facilitate the metastasis and growth of cancer cells49. The metastasis of cancer cells to the specific target organ is highly reliant on the expression of integrins contained in the tumor exosomes50. Upon entering the recipient cells, exosomes may promote conditions that are suitable for the microenvironment formation before metastasis by activating Src phosphorylation and increasing S100 expression. Zhang et al reported that exosomal microRNA could induce microenvironmental PTEN loss in brain tissues, which then resulted in astrocytoma metastasis51.
Increasing evidence indicates that cancer cell-derived exosomes cause complex effects on stromal cells, vascular endothelial cells (VECs), and fibroblasts, which then promote the pathogenesis and development of cancer. Alterations in vascular permeability are indicators of microenvironmental formation before metastasis52. Exosomes with abundant miR-105 from highly metastatic breast cancer cells could inhibit ZO-1 protein expression in endotheliocytes, which then altered the vascular permeability and increased the susceptibility to cancer. This mechanism was attributed to the hepatic metastasis of breast cancer53. Pancreatic cancer exosomes containing integrin β5 and αv could be specifically recognized by Kupffer cells in the hepatic tissues. After phagocytosis of Kupffer cells, exosome-released macrophage migration inhibitory factor (MIF) upregulated the secretion of TGFβ and contributed to the generation of fibronectin by Kupffer cells in the liver. This fibrotic microenvironment could enhance the recruitment of bone marrow-derived macrophages, which finally induced the formation of the microenvironment before hepatic metastasis, as well as the possibility of liver metastasis. Otherwise, strategies that inhibit MIF expression could attenuate the microenvironment formation in the liver before metastasis and thus limit hepatic metastasis of cancer cells54,55.
In 2016, Greening et al reported that exosomes from malignant mesothelioma were involved in the reconstitution and regeneration of blood vessels by enhancing the migration of VECs and fibroblasts56. Similarly, Cui et al reported that exosomes from pulmonary adenocarcinoma may be involved in the angiogenesis of cancer cells by upregulating Ephrin α3 expression in stromal cells in a miR-210-dependent manner57. Moreover, proteins from tumor-derived exosomes, such as Rab3D58, TGF-β159, and LMP160, were reported to promote epithelial-mesenchymal transition (EMT), which then increased the oncogenicity and invasion of cancer cells. In addition to the effects on proliferation of endothelial cells and mesenchymal remodeling, tumor-derived exosomes can also directly regulate the distal lymph node microenvironment, which provided suitable conditions for lymph node metastasis and melanoma growth61. Furthermore, exosomes from stromal cells were reported to affect the biological behaviors of cancer cells. For example, Luga et al revealed that upon trafficking of CD81-positive fibroblast exosomes in breast cancer cells, the fibroblast WNT-11 autocrine protein contributed to the invasion and metastasis of cancer by activating the Wnt-PCP signaling pathway62.
Exosomes involved in the degradation of extracellular matrix (ECM)
ECM is an important tissue barrier for cancer metastasis. Alterations and remodeling of ECM affect the pace of cancer cell invasion. Exosomes from human prostate cancer have varying microRNA contents, such as miR-100-5p, miR-21-5p, and miR-139-5p, which could trigger the expression of matrix metalloproteinases (MMPs), such as MMP2, MMP9 and MMP13. These exosomes are involved in the degradation of ECM and release of growth factors, which finally trigger the invasion and metastasis of cancer cells63. Additionally, tumor exosomes can mediate the direct transmission of MMP13 to recipient cells, which then lead to the degradation of ECM and metastasis of cancer cells64. Moreover, McCready et al showed that invasive carcinoma exosome-released Hsp90 protein could promote cellular migration by activating fibrinogenase65.
Potential of exosomes in cancer therapy
To date, cancer therapy has primarily relied on surgery, radiotherapy, chemotherapy, immunotherapy, and targeted therapy. However, the prognosis remains poor due to the recurrence and metastasis of cancer. Based on the roles of exosomes in the invasion and metastasis of cancer cells, tumor exosomes may be promising new therapeutic candidates for the treatment of cancer and may improve the prognosis and outcomes of cancer therapy.
Tumor-derived exosomes or tumor-related exosomes are considered to be closely related to the pathogenesis and microenvironmental formation of cancer, as the number of exosomes in cancer cells is higher than that of the normal cells66. Therefore, inhibition of the synthesis, release and absolute circulating level of exosomes may serve as an effective treatment regimen for cancer. The formation and secretion of exosomes may be modulated through several approaches. For example, sphingomyelinase 2 could facilitate the synthesis of tumor-derived exosomes. Intravenous injection of an inhibitor of sphingomyelinase 2, GW4869, effectively inhibited the secretion of exosomes in tumor-bearing mice and decreased the metastatic rate of cancer cells67. Chalmin et al reported that 5-N,N-dimethylamiloride could decrease the secretion of exosomes from cancer cells by blocking the H+/Na+ and Na+/Ca2+channels, which subsequently delayed the growth of cancer cells68. In contrast, these phenomena were not observed in PC3 cells69, which indicated that these inhibitory effects might be selective to specific cell types. A few conserved protein families are closely related to the secretion of exosomes, among which Rab27a and Rab27b have been regarded as important mediators of these biological processes70. Bobrie et al showed that silencing of Rab27a and Rab27b by RNA interference may alter the cancer microenvironment and delay the growth and metastasis of cancer cells71. Nevertheless, the release of exosomes was similar in CD9- and MFGE8-positive cells, which indicates that the release of exosomes was partially independent of Rab27a. Similarly, not all regulatory factors could effectively interfere with the release of exosomes. Although the expression of integrin facilitates the migration of cancer cells to specific organs, Mason et al reported confounding results of a phase III trial using cilengitide, an inhibitor of integrin αvβ3 and αvβ5, for treating glioblastoma72. Interestingly, in a recent study, utilization of a hemofiltration system (eg, the Aethlon ADAPT™ system) to capture the exosomes in the circulating system contributed to the decrease of exosome secretion in the circulation, which provided an potential strategy to inhibit the growth and metastasis of cancer cells73.
The unique lipid bilayer structure of the exosome protects it from degradation and RNase damage. Compared with conventional liposome preparations, exosomes showed comparatively lower toxicity and satisfactory tissue tolerance74. Moreover, the integrin expression spectrum contained in these molecules is abundant, which can mediate the migration of exosomes into target organs49. These advantages have attracted increasing interest in developing exosomes as an excellent vector in vivo for the effective delivery of agents for cancer treatment. Exosomes can be used as specific vectors for the intracellular transmission of selective DNAs, miRNAs, non-coding RNAs, and proteins75. For example, an exosome-based delivery platform for adriamycin76 or paclitaxel77 was developed for targeted cancer therapy and shown to have minimal immunogenicity and toxicity. Compared with conventional drug delivery systems, the exosome-based adriamycin delivery system showed higher drug availability with lower toxicity, especially toxicity to the heart. A previous study showed that the let-7 miRNA could inhibit the PI3K signaling pathway in cancer cells, which shares the same target PI3K signaling pathway as that for gefitinib for lung cancer78. The overlap of targets for let-7 miRNA and gefitinib may induce sensitization of these two agents. Given that anti-miR-135 contributed to the sensitivity of lung cancer cells to taxanes, we speculated that delivery of specific miRNAs into specific cells using exosomes, which then increase the sensitivity of cancer cells to chemotherapy, could be a promising approach79. According to previous results, the resistance of pulmonary adenocarcinoma to paclitaxel and cisplatin was related to the loss of PTEN on chromosome24. Therefore, delivery of miR-181a through exosomes into pulmonary adenocarcinoma may increase the sensitivity of the cancer cells to paclitaxel and platinum-based chemotherapy80.
Another advantage for the use of exosomes as a therapeutic vector is their small size. Exosomes can easily pass through various biological barriers, including the blood-brain barrier81.
Extensive studies have been carried out to investigate the roles of exosomes in immunological reactions after Zitvogel et al reported that exosomes are involved in immunological reactions in 19984. Exosomes are now considered a new hot topic in research on vaccine development in cancer treatment. In a previous study, it was reported that after stimulation of cancer-related antigens, DCs could produce exosomes containing specific cancer antigens82. The tumor-derived exosomes and DC-derived exosomes could migrate into specific lymph nodes and then activate CD4+ and CD8+ T cells to trigger anti-tumor immunological reactions. To date, there are indeed some promising reports on the development of cancer vaccines for treating lung cancer. For example, in a clinical trial, TG4010 could extend the survival duration of patients with non-small cell lung cancer (NSCLC) at stages IIIb and IV83. Meanwhile, the EGF vaccination was reported to be effective for the treatment of NSCLC patients84. Additionally, in a recent phase II clinical trial focused on the treatment of NSCLC patients, generation of exosomes by DCs in the presence of tumor-related antigens could extend the progression-free survival of advanced cancer patients with low expression of NKp3085.
Tumor exosomes were reported to facilitate the apoptosis of cancer cells. Cancer cells could deliver survivin into the lung cancer cells through exosomes, which then facilitated the apoptosis of recipient cells by inhibiting cell growth. Survivin-D53A, a dominant-negative mutant survivin, could promote the apoptosis of pulmonary adenocarcinoma86. Some components in vegetal cell-derived exosomes also showed anti-cancer effects. For example, the combination of curcumin and triptolide could increase the apoptosis of ovarian cancer cells87.
Caveats and limitations of exosomes for precision therapy of various cancers
Currently, clinical application of exosomes in cancer therapy is still not possible due to the following major caveats and limitations: i) specific markers for the identification of extracellular vesicles are still lacking; ii) there is currently no standard for the isolation and purification of specific tumor exosomes88; iii) more effective methods are needed to obtain homogenous exosomes; iv) regulatory control of exosome contents and secretion is still poorly understood. Some exosomes could also promote growth and invasion as well as metastasis of cancer cells. For example, several studies confirmed that exosomes could induce complete metastasis of cancer cells50,54. Hoshino et al reported that unconventional secretion of exosome vesicles from multivesicular endosomes (MVE) occurs across a broad set of systems and is increased in cancer, where it promotes aggressive behavior. Analysis of cancer cells identified specialized invasive actin structures called invadopodia as specific and critical docking and secretion sites for CD63- and Rab27a-positive MVE50. Inhibition of invadopodia formation reduced exosome secretion into conditioned media. Addition of purified exosomes or inhibition of exosome biogenesis or secretion significantly affected multiple invadopodia life cycle steps, including invadopodia formation, stabilization, and exocytosis of proteinases. Therefore, exosome cargoes may play a key role in promoting invasive activity and providing in situ signaling feedback. Exosome secretion also controlled cellular invasion through a 3-dimensional matrix. The synergistic interaction between exosome secretion and invadopodia biogenesis strongly suggests that regulation of tumor exosome secretion may play a fundamental role in promoting cancer cell invasiveness50. Pancreatic ductal adenocarcinomas (PDACs) are highly metastatic and have a poor prognosis due to delayed detection. PDAC-derived exosomes were reported to induce liver pre-metastatic niche formation in naive mice and consequently increase liver metastatic burden. Uptake of PDAC-derived exosomes by Kupffer cells caused TGFβ secretion and upregulated fibronectin production by hepatic stellate cells. This fibrotic microenvironment enhanced recruitment of bone marrow-derived macrophages. MIF was substantially higher in PDAC-derived exosomes from stage I PDAC patients who later developed liver metastasis than exosomes from patients whose pancreatic tumors did not progress. When MIF expression in the PDAC-derived exosomes was blocked, liver pre-metastatic niche formation and metastasis were also prevented. These findings suggest that MIF in tumor exosomes may prime the liver for metastasis and may be a prognostic marker for the development of PDAC liver metastasis54. Leal et al reported that tumor-derived exosomes and neutrophils may act cooperatively in cancer-associated thrombosis because the thrombosis in the cancer-bearing mice was faster than that of the normal controls89.
Understanding the underlying and complex mechanisms of exosome-mediated immunosuppression is important for preventing the immune escape of tumor cells and for identifying novel treatments for cancer. DCs and lymphocytes in the tumor microenvironment may modulate immune responses; however, tumor-derived exosomes may mediate the death receptor pathway to induce CD8+ T lymphocyte apoptosis. In addition, these molecules could mediate T lymphocyte imbalance by regulating the proliferation of T lymphocytes and inhibiting the proliferation of effector T lymphocytes, which then inhibited the immune function of the tumor microenvironment90,91. As a means of cellular communication, tumor-derived exosomes can inhibit the immune function of the receptor cells or trigger the distribution of immunologic receptors and ligands of the receptor cells with a similar pattern to that of oncocytes. Thereafter, they may interfere with immune therapies via sequestration of therapeutic antibodies or elimination of vaccine-induced or adoptively transferred immune effector cells92. In a recent study, tumor-derived exosomes were shown to activate the transfer of epidermal growth factor receptor to macrophages in the hosts, inhibit innate immunity and induce immunocompromise93.
Therefore, in contrast to exosomes from other sources used to deliver therapeutic agents, tumor exosomes may be a double-edged sword when used as a therapeutic tool for cancer treatment. Full elucidation of the formation, secretion, and networking function of tumor exosomes is urgently needed for the realization of this attractive and promising strategy for cancer therapy. More extensive clinical or animal studies with large sample sizes are needed in the future to investigate the features and safety of tumor exosomes serving as either a target or a vector for genes and/or drugs.
Conclusion and perspectives
In the future, we will need to broaden our understanding of the biological features and biochemical characteristics as well as the functional roles of tumor exosomes in the pathogenesis and development of cancer. Only when the specificity, efficacy, functionality, toxicity, and safety of exosomes have been fully elucidated can tumor exosomes serve as promising candidates for anti-cancer therapy and be developed as new strategies for precision therapy of various cancers.
References
Kreger BT, Dougherty AL, Greene KS, Cerione RA, Antonyak MA . Microvesicle cargo and function changes upon induction of cellular transformation. J Biol Chem 2016; 291: 19774–85.
Trams EG, Lauter CJ, Salem N Jr, Heine U . Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta 1981; 645: 63–70.
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C . Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 1987; 262: 9412–20.
Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 1998; 4: 594–600.
Pfeffer SR . A prize for membrane magic. Cell 2013; 155: 1203–6.
Kaiser CA, Schekman R . Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell 1990; 61: 723–33.
Balch WE, Dunphy WG, Braell WA, Rothman JE . Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell 1984; 39: 405–16.
Hata Y, Slaughter CA, Sudhof TC . Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature 1993; 366: 347–51.
Chaput N, Thery C . Exosomes: immune properties and potential clinical implementations. Semin Immunopathol 2011; 33: 419–40.
Harada T, Yamamoto H, Kishida S, Kishida M, Awada C, Takao T, et al. Wnt5b-associated exosomes promote cancer cell migration and proliferation. Cancer Sci 2017; 108: 42–52.
Hendrix A, Hume AN . Exosome signaling in mammary gland development and cancer. Int J Dev Biol 2011; 55: 879–87.
Hewson C, Morris KV . Form and function of exosome-associated long non-coding RNAs in cancer. Curr Top Microbiol Immunol 2016; 394: 41–56.
Gilligan KE, Dwyer RM . Engineering exosomes for cancer therapy. Int J Mol Sci 2017; 18. pii: E1122.
Javeed N, Mukhopadhyay D . Exosomes and their role in the micro-/macro-environment: a comprehensive review. J Biomed Res 2017; 31: 386–94.
Steinbichler TB, Dudas J, Riechelmann H, Skvortsova II . The role of exosomes in cancer metastasis. Semin Cancer Biol 2017; 44: 170–81.
Wang J, Sun X, Zhao J, Yang Y, Cai X, Xu J, et al. Exosomes: a novel strategy for treatment and prevention of diseases. Front Pharmacol 2017; 8: 300.
Weidle UH, Birzele F, Kollmorgen G, Ruger R . The multiple roles of exosomes in metastasis. Cancer Genomics Proteomics 2017; 14: 1–15.
Paggetti J, Haderk F, Seiffert M, Janji B, Distler U, Ammerlaan W, et al. Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood 2015; 126: 1106–17.
Palazzolo G, Albanese NN, DI CG, Gygax D, Vittorelli ML, Pucci-Minafra I . Proteomic analysis of exosome-like vesicles derived from breast cancer cells. Anticancer Res 2012; 32: 847–60.
Pan J, Ding M, Xu K, Yang C, Mao LJ . Exosomes in diagnosis and therapy of prostate cancer. Oncotarget 2017; 8: 97693–700.
Panagiotara A, Markou A, Lianidou ES, Patrinos GP, Katsila T . Exosomes: a cancer theranostics road map. Public Health Genomics 2017; 20: 116–25.
Gangoda L, Liem M, Ang CS, Keerthikumar S, Adda CG, Parker BS, et al. Proteomic profiling of exosomes secreted by breast cancer cells with varying metastatic potential. Proteomics 2017; 17. doi: 10.1002/pmic.201600370.
Keerthikumar S, Gangoda L, Liem M, Fonseka P, Atukorala I, Ozcitti C, et al. Proteogenomic analysis reveals exosomes are more oncogenic than ectosomes. Oncotarget 2015; 6: 15375–96.
Keerthikumar S, Chisanga D, Ariyaratne D, Al SH, Anand S, Zhao K, et al. ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol 2016; 428: 688–92.
Villarroya-Beltri C, Baixauli F, Gutierrez-Vazquez C, Sanchez-Madrid F, Mittelbrunn M . Sorting it out: regulation of exosome loading. Semin Cancer Biol 2014; 28: 3–13.
Cho JA, Park H, Lim EH, Lee KW . Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int J Oncol 2012; 40: 130–8.
Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM . Exosome mediated communication within the tumor microenvironment. J Control Release 2015; 219: 278–94.
Nabet BY, Qiu Y, Shabason JE, Wu TJ, Yoon T, Kim BC, et al. Exosome RNA unshielding couples stromal activation to pattern recognition receptor signaling in cancer. Cell 2017; 170: 352–66.
Naito Y, Yoshioka Y, Yamamoto Y, Ochiya T . How cancer cells dictate their microenvironment: present roles of extracellular vesicles. Cell Mol Life Sci 2017; 74: 697–713.
Whiteside TL . Exosome and mesenchymal stem cell cross-talk in the tumor microenvironment. Semin Immunol 2017. pii: S1044-5323(17)30010-6.
Bhome R, Goh RW, Bullock MD, Pillar N, Thirdborough SM, Mellone M, et al. Exosomal microRNAs derived from colorectal cancer-associated fibroblasts: role in driving cancer progression. Aging (Albany NY) 2017; 9: 2666–94.
Devhare PB, Ray RB . Extracellular vesicles: Novel mediator for cell to cell communications in liver pathogenesis. Mol Aspects Med 2017. pii: S0098-2997(17)30089-4.
Shenoy GN, Loyall JL, Maguire O, Iyer V, Kelleher RJ, Minderman H, et al. Exosomes associated with human ovarian tumors harbor a reversible checkpoint of T cell responses. Cancer Immunol Res 2018; 6: 236–47.
Wu K, Xing F, Wu SY, Watabe K . Extracellular vesicles as emerging targets in cancer: recent development from bench to bedside. Biochim Biophys Acta 2017; 1868: 538–63.
Wang J, Yeung BZ, Cui M, Peer CJ, Lu Z, Figg WD, et al. Exosome is a mechanism of intercellular drug transfer: application of quantitative pharmacology. J Control Release 2017; 268: 147–58.
Sueta A, Yamamoto Y, Tomiguchi M, Takeshita T, Yamamoto-Ibusuki M, Iwase H . Differential expression of exosomal miRNAs between breast cancer patients with and without recurrence. Oncotarget 2017; 8: 69934–44.
Patel GK, Khan MA, Bhardwaj A, Srivastava SK, Zubair H, Patton MC, et al. Exosomes confer chemoresistance to pancreatic cancer cells by promoting ROS detoxification and miR-155-mediated suppression of key gemcitabine-metabolising enzyme, DCK. Br J Cancer 2017; 116: 609–19.
Hanson PI, Cashikar A . Multivesicular body morphogenesis. Annu Rev Cell Dev Biol 2012; 28: 337–62.
Vlassov AV, Magdaleno S, Setterquist R, Conrad R . Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta 2012; 1820: 940–8.
Romano G, Kwong LN . miRNAs, melanoma and microenvironment: an intricate network. Int J Mol Sci 2017; 18. pii: E2354.
Takano Y, Masuda T, Iinuma H, Yamaguchi R, Sato K, Tobo T, et al. Circulating exosomal microRNA-203 is associated with metastasis possibly via inducing tumor-associated macrophages in colorectal cancer. Oncotarget 2017; 8: 78598–613.
Fan Q, Yang L, Zhang X, Peng X, Wei S, Su D, et al. The emerging role of exosome-derived non-coding RNAs in cancer biology. Cancer Lett 2018; 414: 107–15.
Umezu T, Tadokoro H, Azuma K, Yoshizawa S, Ohyashiki K, Ohyashiki JH . Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood 2014; 124: 3748–57.
Umezu T, Imanishi S, Azuma K, Kobayashi C, Yoshizawa S, Ohyashiki K, et al. Replenishing exosomes from older bone marrow stromal cells with miR-340 inhibits myeloma-related angiogenesis. Blood Adv 2017; 1: 812–23.
Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol 2008; 10: 619–24.
Demory Beckler M, Higginbotham JN, Franklin JL, Ham AJ, Halvey PJ, Imasuen IE, et al. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol Cell Proteomics 2013; 12: 343–55.
Gezer U, Ozgur E, Cetinkaya M, Isin M, Dalay N . Long non-coding RNAs with low expression levels in cells are enriched in secreted exosomes. Cell Biol Int 2014; 38: 1076–9.
Le MT, Hamar P, Guo C, Basar E, Perdigao-Henriques R, Balaj L, et al. miR-200-containing extracellular vesicles promote breast cancer cell metastasis. J Clin Invest 2014; 124: 5109–28.
Hood JL, San RS, Wickline SA . Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res 2011; 71: 3792–801.
Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015; 527: 329–35.
Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 2015; 527: 100–4.
Psaila B, Lyden D . The metastatic niche: adapting the foreign soil. Nat Rev Cancer 2009; 9: 285–93.
Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014; 25: 501–15.
Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 2015; 17: 816–26.
Tomasetti M, Lee W, Santarelli L, Neuzil J . Exosome-derived microRNAs in cancer metabolism: possible implications in cancer diagnostics and therapy. Exp Mol Med 2017; 49: e285.
Greening DW, Ji H, Chen M, Robinson BW, Dick IM, Creaney J, et al. Secreted primary human malignant mesothelioma exosome signature reflects oncogenic cargo. Sci Rep 2016; 6: 32643.
Cui H, Seubert B, Stahl E, Dietz H, Reuning U, Moreno-Leon L, et al. Tissue inhibitor of metalloproteinases-1 induces a pro-tumourigenic increase of miR-210 in lung adenocarcinoma cells and their exosomes. Oncogene 2015; 34: 3640–50.
Yang J, Liu W, Lu X, Fu Y, Li L, Luo Y . High expression of small GTPase Rab3D promotes cancer progression and metastasis. Oncotarget 2015; 6: 11125–38.
Ko H, Jeon H, Lee D, Choi HK, Kang KS, Choi KC . Sanguiin H6 suppresses TGF-beta induction of the epithelial-mesenchymal transition and inhibits migration and invasion in A549 lung cancer. Bioorg Med Chem Lett 2015; 25: 5508–13.
Hood JL, Pan H, Lanza GM, Wickline SA ; Consortium for Translational Research in Advanced Imaging and Nanomedicine (C-TRAIN). Paracrine induction of endothelium by tumor exosomes. Lab Invest 2009; 89: 1317–28.
Aga M, Bentz GL, Raffa S, Torrisi MR, Kondo S, Wakisaka N, et al. Exosomal HIF1alpha supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes. Oncogene 2014; 33: 4613–22.
Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 2012; 151: 1542–56.
Sanchez CA, Andahur EI, Valenzuela R, Castellon EA, Fulla JA, Ramos CG, et al. Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget 2016; 7: 3993–4008.
You Y, Shan Y, Chen J, Yue H, You B, Shi S, et al. Matrix metallo proteinase 13-containing exosomes promote nasopharyngeal carcinoma metastasis. Cancer Sci 2015; 106: 1669–77.
McCready J, Sims JD, Chan D, Jay DG . Secretion of extracellular hsp90alpha via exosomes increases cancer cell motility: a role for plasminogen activation. BMC Cancer 2010; 10: 294.
Jenjaroenpun P, Kremenska Y, Nair VM, Kremenskoy M, Joseph B, Kurochkin IV . Characterization of RNA in exosomes secreted by human breast cancer cell lines using next-generation sequencing. PeerJ 2013; 1: e201.
Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A 2012; 109: E2110–6.
Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest 2010; 120: 457–71.
Phuyal S, Hessvik NP, Skotland T, Sandvig K, Llorente A . Regulation of exosome release by glycosphingolipids and flotillins. FEBS J 2014; 281: 2214–27.
Hendrix A, De Wever O . Rab27 GTPases distribute extracellular nanomaps for invasive growth and metastasis: implications for prognosis and treatment. Int J Mol Sci 2013; 14: 9883–92.
Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, et al. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res 2012; 72: 4920–30.
Mason WP . End of the road: confounding results of the CORE trial terminate the arduous journey of cilengitide for glioblastoma. Neuro Oncol 2015; 17: 634–5.
Marleau AM, Chen CS, Joyce JA, Tullis RH . Exosome removal as a therapeutic adjuvant in cancer. J Transl Med 2012; 10: 134.
Lai RC, Yeo RW, Tan KH, Lim SK . Exosomes for drug delivery - a novel application for the mesenchymal stem cell. Biotechnol Adv 2013; 31: 543–51.
Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res 2014; 24: 766–9.
Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014; 35: 2383–90.
Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, et al. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res 2015; 32: 2003–14.
Sebio A, Pare L, Paez D, Salazar J, Gonzalez A, Sala N, et al. The LCS6 polymorphism in the binding site of let-7 microRNA to the KRAS 3'-untranslated region: its role in the efficacy of anti-EGFR-based therapy in metastatic colorectal cancer patients. Pharmacogenet Genomics 2013; 23: 142–7.
Holleman A, Chung I, Olsen RR, Kwak B, Mizokami A, Saijo N, et al. miR-135a contributes to paclitaxel resistance in tumor cells both in vitro and in vivo. Oncogene 2011; 30: 4386–98.
Li H, Zhang P, Sun X, Sun Y, Shi C, Liu H, et al. MicroRNA-181a regulates epithelial-mesenchymal transition by targeting PTEN in drug-resistant lung adenocarcinoma cells. Int J Oncol 2015; 47: 1379–92.
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ . Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 2011; 29: 341–5.
Romagnoli GG, Zelante BB, Toniolo PA, Migliori IK, Barbuto JA . Dendritic cell-derived exosomes may be a tool for cancer immunotherapy by converting tumor cells into immunogenic targets. Front Immunol 2014; 5: 692.
Rotonda C, Anota A, Mercier M, Bastien B, Lacoste G, Limacher JM, et al. Impact of TG4010 vaccine on health-related quality of life in advanced non-small-cell lung cancer: results of a Phase IIB clinical trial. PLoS One 2015; 10: e0132568.
Crombet Ramos T, Rodriguez PC, Neninger Vinageras E, Garcia Verdecia B, Lage Davila A . CIMAvax EGF (EGF-P64K) vaccine for the treatment of non-small-cell lung cancer. Expert Rev Vaccines 2015; 14: 1303–11.
Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 2016; 5: e1071008.
Yu M, Peng X, Lu Y, Huang M . Inhibition function of dominant-negative mutant gene survivin-D53A to SPC-A1 lung adenocarcinoma xenograft in nude mice models. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2015; 32: 624–8.
Cai YY, Lin WP, Li AP, Xu JY . Combined effects of curcumin and triptolide on an ovarian cancer cell line. Asian Pac J Cancer Prev 2013; 14: 4267–71.
Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A 2016; 113: E968–77.
Leal AC, Mizurini DM, Gomes T, Rochael NC, Saraiva EM, Dias MS, et al. Tumor-derived exosomes induce the formation of neutrophil extracellular traps: implications for the establishment of cancer-associated thrombosis. Sci Rep 2017; 7: 6438.
Whiteside TL . The effect of tumor-derived exosomes on immune regulation and cancer immunotherapy. Future Oncol 2017; 28: 2583–92.
Gao L, Wang L, Dai T, Jin K, Zhang Z, Wang S, et al. Tumor-derived exosomes antagonize innate antiviral immunity. Nat Immunol 2018. doi: 10.1038/s41590-017-0043-5.
Peng P, Yan Y, Keng S . Exosomes in the ascites of ovarian cancer patients: origin and effects on anti-tumor immunity. Oncol Rep 2011; 25: 749–62.
Miller IV, Grunewald TG . Tumour-derived exosomes: tiny envelopes for big stories. Biol Cell 2015; 107: 287–305.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81773224), the Scientific Program of Changzhou (CE20175025) and the Scientific and Technology Program of Nanjing Medical University (2015NJMU172).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sun, W., Luo, Jd., Jiang, H. et al. Tumor exosomes: a double-edged sword in cancer therapy. Acta Pharmacol Sin 39, 534–541 (2018). https://doi.org/10.1038/aps.2018.17
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2018.17
Keywords
This article is cited by
-
Exosomal miRNAs—a diagnostic biomarker acting as a guiding light in the diagnosis of prostate cancer
Functional & Integrative Genomics (2023)
-
Cancer-derived small extracellular vesicles: emerging biomarkers and therapies for pancreatic ductal adenocarcinoma diagnosis/prognosis and treatment
Journal of Nanobiotechnology (2022)
-
Nanotechnological engineering of extracellular vesicles for the development of actively targeted hybrid nanodevices
Cell & Bioscience (2022)
-
The prognostic and predictive values of differential expression of exosomal receptor tyrosine kinases and associated with the PI3K/AKT/mTOR signaling in breast cancer patients undergoing neoadjuvant chemotherapy
Clinical and Translational Oncology (2022)
-
Cancer Stem Cells and the Tumor Microenvironment: Targeting the Critical Crosstalk through Nanocarrier Systems
Stem Cell Reviews and Reports (2022)