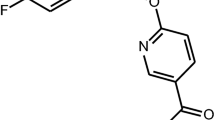
Abstract
Aim:
Zolpidem is a non-benzodiazepine agonist at benzodiazepine binding site in GABAA receptors, which is increasingly prescribed. Recent studies suggest that prolonged zolpidem treatment induces tolerance. The aim of this study was to explore the adaptive changes in GABAA receptors following short and long-term exposure to zolpidem in vitro.
Methods:
Human embryonic kidney (HEK) 293 cells stably expressing recombinant α1β2γ2s GABAA receptors were exposed to zolpidem (1 and 10 μmol/L) for short-term (2 h daily for 1, 2, or 3 consecutive days) or long-term (continuously for 48 h). Radioligand binding studies were used to determine the parameters of [3H]flunitrazepam binding sites.
Results:
A single (2 h) or repeated (2 h daily for 2 or 3 d) short-term exposure to zolpidem affected neither the maximum number of [3H]flunitrazepam binding sites nor the affinity. In both control and short-term zolpidem treated groups, addition of GABA (1 nmol/L–1 mmol/L) enhanced [3H]flunitrazepam binding in a concentration-dependent manner. The maximum enhancement of [3H]flunitrazepam binding in short-term zolpidem treated group was not significantly different from that in the control group. In contrast, long-term exposure to zolpidem resulted in significantly increase in the maximum number of [3H]flunitrazepam binding sites without changing the affinity. Furthermore, long-term exposure to zolpidem significantly decreased the ability of GABA to stimulate [3H]flunitrazepam binding.
Conclusion:
The results suggest that continuous, but not intermittent and short-term, zolpidem-exposure is able to induce adaptive changes in GABAA receptors that could be related to the development of tolerance and dependence.
Similar content being viewed by others
Introduction
Gamma-aminobutyric acid type A (GABAA) receptors are ligand gated ion channels activated physiologically by the main inhibitory neurotransmitter γ-aminobutyric acid (GABA). Functional GABAA receptors are formed by five subunits derived from seven receptor subunit families (α1-6, β1-3, γ1-3, δ, ɛ, θ, and π). Different subunit isoforms are expressed with regional specificity and in a cell-type specific manner1. In the central nervous system, the most common receptor form is comprised of the α1, β2, and γ2 subunits, with a defined stoichiometry of 2α:2β:1γ2.
Imidazopyridine zolpidem is a non-benzodiazepine hypnotic that exerts its effects via the benzodiazepine binding site on GABAA receptors. Zolpidem has a very high affinity for receptors containing the α1 subunit, has an intermediate affinity for receptors that contain α2 or α3, and does not interact with GABAA receptors consisting of the α5 subunit3,4,5,6. In addition to its sedative effects, zolpidem has considerable anticonvulsant activity7,8. Moreover, recent studies provide a rationale for further investigations of its potential in the treatment of basal ganglia disorders9.
Benzodiazepines are widely used clinically to obtain one of the following major effects: decrease in sleep latency, reduction of anxiety, antiepileptic action or muscle relaxation. They are positive allosteric modulators of GABAA receptors. Benzodiazepines bind to a binding pocket on the α/γ subunit interface. In general, benzodiazepines are safe and effective for short-term treatment. On the other hand, due to the development of tolerance and potential for dependence, the appropriateness of benzodiazepines for long-term use is controversial.
It has been supposed that drugs with a high selectivity for α1 containing receptors have fewer side effects compared to classical benzodiazepines. Although chemically different than benzodiazepines, zolpidem elicited many side effects similar to that of benzodiazepines. Vlainić and Peričić (2009)10 demonstrated development of anticonvulsant and sedative tolerance after repeated (10 days) zolpidem treatment in mice. Similar results were obtained in rats11. Several studies have also suggested that zolpidem has a significant risk of abuse and dependence in humans12.
In their study, Vlainić and colleagues (2010)13 showed that a 2-day zolpidem (10 μmol/L) treatment enhances the number of recombinant α1β2γ2s GABAA receptors and produces functional uncoupling between GABA and benzodiazepine binding site. Moreover, the observed changes are not substantially different from those detected after prolonged exposure of these cells to high doses of the classical benzodiazepine, diazepam14. Despite of many studies, the molecular mechanisms involved in the development of tolerance to the actions of benzodiazepines remain unknown15,16,17. The aim of our study was to explore the molecular mechanisms induced by zolpidem treatment using radioligand binding assays.
Materials and methods
Cell culture
The human embryonic kidney (HEK) 293 cell line stably expressing the α1β2γ2S subtype of GABAA receptor was kindly donated by Dr David GRAHAM (Sanofi-Synthélabo Research, France). The cells were maintained in 75-cm2 flasks at 37 °C in humidified air with 5% CO2 according to standard cell culture techniques. The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum, 2 mmol/L L-glutamine, 100 units/mL penicillin and 100 μg/mL streptomycin.
Drugs
Zolpidem [N,N,6-trimethyl-2-(4-methylphenyl)-imidazo(1,2-a)pyridine-3-acetamide] was a generous gift from Pliva (Zagreb, Croatia). [3H]flunitrazepam (specific activity 87 Ci/mmol) was purchased from Amersham Biosciences UK Ltd. Culture medium, antibiotics, fetal bovine serum and other chemicals were supplied from Invitrogen/Gibco (Grand Island, NY, USA).
Drug treatment
Cells were seeded onto new flasks and grown for 3 d prior to exposure to drugs. We had four different treatment regimens:
Continuous zolpidem treatment: on the initial day, the medium was replaced with a fresh medium containing zolpidem (final concentrations 1 and 10 μmol/L) for 48 h in the presence of 1 μmol/L GABA.
Single dose of zolpidem (final concentrations 1 and 10 μmol/L): the medium containing zolpidem was replaced with fresh medium following a 2-h treatment period. The cells were grown in the presence of 1 μmol/L GABA.
Zolpidem treatment for two consecutive days: the medium containing zolpidem (final concentrations 1 and 10 μmol/L) was replaced after a 2-h treatment with fresh medium. The same procedure (treatment/fresh medium) was repeated the next day. The cells were grown in the presence of 1 μmol/L GABA.
Zolpidem treatment for three consecutive days: zolpidem (final concentrations 1 and 10 μmol/L) was added to the medium for a 2-h treatment period and then replaced with fresh medium. The same procedure was repeated for three consecutive days. The cells were grown in the presence of 1 μmol/L GABA.
Zolpidem and GABA were dissolved in distilled water. The control cells were treated with corresponding vehicle in the presence of 1 μmol/L GABA.
Radioligand binding studies
Preparation of the membranes
Membranes from stably transfected HEK 293 cells were prepared mainly as described by Peričić et al (2005)18. Briefly, the cells were washed with phosphate-buffer saline (PBS), scraped from the flasks into ice-cold PBS, and centrifuged at 12 000×g for 12 min. The cell pellet was homogenized in 50 mmol/L Tris-citrate buffer at pH 7.4 by 10 strokes (up and down) at 1250 r/min using a teflon pestle and glass homogenizer. The cells were then centrifuged at 200 000×g for 20 min. The same procedure (re-suspension/ centrifugation) was repeated two more times. Finally, the pellet was re-suspended and stored in aliquots at -20 °C. The cell membrane suspension was centrifuged once more on the day of the [3H]flunitrazepam binding assay at 200 000×g for 20 min.
[3H]flunitrazepam binding assay
Aliquots of the cell membrane preparation (∼100 μg protein) were incubated in a 50 mmol/L Tris-citrate buffer supplemented with 150 mmol/L NaCl at 4 °C for 90 min with the addition of varying concentrations of non-radioactive flunitrazepam (ten final concentrations in the range of 0.4–50 nmol/L) and a fixed concentration (1 nmol/L) of [3H]flunitrazepam. In stimulation studies, varying concentrations of GABA (1 nmol/L–1 mmol/L) were incubated with [3H]flunitrazepam (1 nmol/L). Non-specific binding was determined in the presence of 100 μmol/L diazepam. Total assay volume of all binding studies was 0.5 mL. The radioactivity bound to the membranes was counted on a β-scintillation counter (Perkin Elmer, Wallace 1409DSA) after a rapid vacuum filtration on Whatman GF/C filters.
Using bovine serum albumin as a standard, the protein concentration was determined in 10 μL samples of each membrane suspension.
Statistical analysis
The analysis of binding data was performed using the GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA, USA). The values dissociation constant (Kd) and maximum number of (3H)flunitrazepam binding sites (Bmax) were obtained by nonlinear regression using the equation for a hyperbola (one binding site): Y=Bmax×X/(Kd+X), where Kd is the concentration of ligand required to reach half-maximal binding and Bmax is the maximum number of binding sites. The percentage of change in [3H]flunitrazepam binding produced by GABA was defined as (specific binding in the presence of GABA/specific binding in the absence of GABA)×100. The enhancement curves, analyzed using the sigmoidal equation, determined the values for half-maximum (EC50) and the maximum enhancement (Emax, defined as absolute difference between the top and bottom plateau) of GABA-induced [3H]flunitrazepam binding.
Statistical evaluation was performed with one-way analysis of variance (ANOVA) followed by a post-hoc Newman-Keuls multiple comparison test. All data are expressed as the mean±SEM of at least three independent experiments performed in duplicate. P-values of less than 0.05 were considered significant.
Results
The effect of long-term zolpidem treatment on [3H]flunitrazepam binding to membranes from HEK 293 cells stably transfected with α1β2γ2s subunits of GABAA receptors
Long-term zolpidem treatment (1 and 10 μmol/L for 48 h in the presence of 1 μmol/L GABA) induced an up-regulation of benzodiazepine binding sites at recombinant α1β2γ2s GABAA receptors. As shown in Figure 1, zolpidem up-regulated the maximum number of benzodiazepine binding sites (Bmax) by 35% and 104% (Bmax values were as follows: control group, 2.95±0.24 pmol/mg protein; 1 μmol/L zolpidem treatment, 3.99±0.51 pmol/mg protein; and 10 μmol/L zolpidem treatment, 6.03±0.18 pmol/mg protein). One-way ANOVA revealed significant differences between these groups [F(2,15)=15.75; P<0.0003], indicating that zolpidem treatment had a significant effect on the maximum number of benzodiazepine binding sites, whereas their affinity remained unchanged. Kd values were as follows: control group, 2.56±0.32 nmol/L; 1 μmol/L zolpidem treatment, 2.75±0.44 nmol/L; and 10 μmol/L zolpidem treatment, 2.62±0.11 nmol/L.
The effect of zolpidem treatment (1 and 10 μmol/L, 48 h) on the Scatchard plot (A), saturation isotherms (B), maximum number (C; Bmax) and dissociation constant (D; Kd) of [3H]flunitrazepam binding sites on the membranes of HEK 293 cells stably transfected with α1β2γ2s subunits of GABAA receptors. Cell membranes were prepared and incubated with increasing concentrations of non-radioactive flunitrazepam (0.3–50 nmol/L) in the presence of 1 nmol/L [3H]flunitrazepam. Bmax and Kd values were obtained by nonlinear regression using GraphPad Prism. Mean±SEM. n=3. bP<0.05 and cP<0.01 versus control group (ANOVA followed by the Newman-Keuls test).
The effect of short-term 1 μmol/L zolpidem treatment on [3H]flunitrazepam binding to membranes from HEK 293 cells stably transfected with α1β2γ2s subunits of GABAA receptors
The cells were treated with 1 μmol/L zolpidem once for 2 h, or for a 2-h period per day during two or three consecutive days in the presence of 1 μmol/L GABA. As shown in Figure 2, intermittent short-term exposure of cells to 1 μmol/L zolpidem did not induce adaptive changes in the maximum number of [3H]flunitrazepam binding sites at GABAA receptors. The values for the maximum number of benzodiazepine binding sites were as follows: control group, 2.43±0.34 pmol/mg protein; one-time, 2 h zolpidem treatment, 2.12±0.26 pmol/mg protein; 2 h zolpidem treatment for two consecutive days, 2.11±0.45 pmol/mg protein; and 2 h zolpidem treatment for three consecutive days, 1.92±0.12 pmol/mg protein. One-way ANOVA did not reveal significant differences between these groups. In addition, the affinity of benzodiazepine binding sites was not affected with zolpidem treatment: control group, 2.87±0.34 nmol/L; one-time, 2 h zolpidem treatment, 2.66±0.26 nmol/L; 2 h zolpidem treatment for two consecutive days, 2.66±0.23 nmol/L; and 2 h zolpidem treatment for three consecutive days, 2.98±0.21 nmol/L).
The effect of short-term zolpidem treatment (1 μmol/L for 2 h during one, two or three consecutive days) on the saturation isotherms (A), maximum number (B; Bmax) and dissociation constant (C; Kd) of [3H]flunitrazepam binding sites on the membranes of HEK 293 cells stably transfected with α1β2γ2s subunits of GABAA receptors. Cell membranes were prepared and incubated with increasing concentrations of non-radioactive flunitrazepam (0.3–50 nmol/L) in the presence of 1 nmol/L [3H]flunitrazepam. Bmax and Kd values were obtained by nonlinear regression using GraphPad Prism. The results are expressed as mean±SEM. n=3. Statistical analysis showed no significant changes among the groups (ANOVA followed by the Newman-Keuls test).
The effect of short-term 10 μmol/L zolpidem on [3H]flunitrazepam binding to membranes from HEK 293 cells stably transfected with α1β2γ2s subunits of GABAA receptors
The cells were treated with 10 μmol/L zolpidem once for 2 h, or for a 2-h period per day during two or three consecutive days in the presence of 1 μmol/L GABA. As shown in Figure 3 and indicated by one-way ANOVA, there is no significant difference among these groups. The intermittent short-term zolpidem treatment (2 h once and during two or three consecutive days) did not affect [3H]flunitrazepam binding parameters at recombinant α1β2γ2s GABAA receptors stably expressed in HEK 293 cells (P<0.001, ANOVA and Newman-Keuls test). Bmax values for the control group were 2.45±0.24 pmol/mg protein; one-time, 2 h zolpidem treatment, 1.77±0.11 pmol/mg protein; 2 h zolpidem treatment for two consecutive days, 2.22±0.07 pmol/mg protein; and 2 h zolpidem treatment for three consecutive days, 1.91±0.20 pmol/mg protein. The affinity of benzodiazepine binding sites was not affected with zolpidem treatment. The dissociation constant for the control group was 3.75±0.62 nmol/L; one-time, 2 h zolpidem treatment, 4.41±0.36 nmol/L; 2 h zolpidem treatment for two consecutive days, 3.86±0.22 nmol/L; and 2 h zolpidem treatment for three consecutive days, 4.45±0.69 nmol/L.
The effect of short-term zolpidem treatment (10 μmol/L for 2 h during one, two or three consecutive days) on the saturation isotherms (A), maximum number (B; Bmax) and dissociation constant (C; Kd) of [3H]flunitrazepam binding sites on the membranes of HEK 293 cells stably transfected with α1β2γ2s subunits of GABAA receptors. Cell membranes were prepared and incubated with increasing concentrations of non-radioactive flunitrazepam (0.3–50 nmol/L) in the presence of 1 nmol/L [3H]flunitrazepam. Bmax and Kd values were obtained by nonlinear regression using GraphPad Prism. The results are expressed as mean±SEM. n=3. Statistical analysis showed no significant changes among the groups (ANOVA followed by the Newman-Keuls test).
The effect of long-term zolpidem treatment (1 and 10 μmol/L) on GABA-induced enhancement of [3H]flunitrazepam binding to membranes of HEK 293 cells stably transfected with α1β2γ2s subunits of GABAA receptors
Long-term zolpidem treatment (1 and 10 μmol/L) of HEK 293 cells enhanced basal [3H]flunitrazepam binding to the same level as that observed in the maximum number of binding sites for benzodiazepines (Bmax). The addition of GABA (1 nmol/L–1 mmol/L) to membranes obtained from control and zolpidem pre-treated cells enhanced [3H]flunitrazepam binding in a concentration-dependent manner. We present the data as the percentage of their own basal values and as the maximum enhancement (Emax) of [3H]flunitrazepam binding by GABA to better see the differences in the intensity of GABA-induced enhancement of [3H]flunitrazepam binding (Figure 4). The maximum enhancement (Emax) of [3H]flunitrazepam binding produced by GABA in the control group was 79.3%±3.2%, indicating that the GABA binding site was functionally coupled to the benzodiazepine binding site. In the group treated with 1 μmol/L zolpidem, the maximum enhancement of [3H]flunitrazepam binding produced by GABA was significantly lower (55.1%±7.9%). Moreover, in the group treated with 10 μmol/L zolpidem, the maximum enhancement of [3H]flunitrazepam binding was even lower (44.2%±7.6%). These results indicate that allosteric interactions between GABA and benzodiazepine binding sites in zolpidem treated groups were uncoupled by 31% and 45%. One-way ANOVA indicated the significant difference [F(2,15)=12.51; P<0.0009] between the analyzed groups. This difference was confirmed by Newman-Keuls test (control group versus group treated with 1 μmol/L zolpidem P<0.05; control group versus group treated with 10 μmol/L zolpidem P<0.001). As shown by analysis of enhancement curves, the concentrations of GABA that produced a half-maximum enhancement of [3H]flunitrazepam binding (EC50) were not different between the control group and the zolpidem-treated groups.
The effect of zolpidem treatment (1 and 10 μmol/L, 48 h) on GABA potentiation of [3H]flunitrazepam binding to membranes of HEK 293 cells stably transfected with α1β2γ2s subunits of GABAA receptors. Data are expressed as percent of their own basal values (A) and as the maximum GABA-induced enhancements (B; Emax) of [3H]flunitrazepam binding. The points and bars are mean±SEM. n=3–5. cP<0.01 versus control group (ANOVA and Newman-Keuls test).
The effect of short-term 1 μmol/L zolpidem treatment on GABA-induced enhancement of [3H]flunitrazepam binding to membranes of HEK 293 cells stably transfected with α1β2γ2s subunits of GABAA receptors
The addition of GABA (1 nmol/L–1 mmol/L) to membranes obtained from control and zolpidem pre-treated cells enhanced [3H]flunitrazepam binding in a concentration-dependent manner. The data are presented as a percentage of their own basal values and as the maximum enhancement (Emax) of [3H]flunitrazepam binding (Figure 5). In the control group, the maximum enhancement of [3H]flunitrazepam binding produced by GABA was 80.2%±3.6%, indicating that the GABA binding sites were functionally coupled to benzodiazepine binding sites. In the short-term zolpidem treated groups, the maximum enhancements were not significantly different from the one produced in the control group (Emax values were: one-time, 2 h zolpidem treatment, 69.9%±3.8%; 2 h zolpidem treatment for two consecutive days, 67.1%±1.9%; and 2 h zolpidem treatment for three consecutive days, 71.4%±9.4%). The analysis of enhancement curves did not reveal differences in the concentrations of GABA that produced a half-maximum enhancement of [3H]flunitrazepam binding (EC50) in control and zolpidem treated groups.
The effect of short-term zolpidem (1 μmol/L for 2 h during one, two or three consecutive days) treatment on [3H]flunitrazepam binding to membranes from HEK 293 cells stably transfected with α1β2γ2s subunits of GABAA receptors. Data are expressed as percent of their own basal values (A) and as the maximum GABA-induced enhancements (B; Emax) of [3H]flunitrazepam binding. The points and bars are mean±SEM. n=3. Statistical analysis showed no significant change among the groups (ANOVA followed by the Newman-Keuls test).
The effect of short-term 10 μmol/L zolpidem treatment on GABA-induced enhancement of [3H]flunitrazepam binding to membranes of HEK 293 cells stably transfected with α1β2γ2s subunits of GABAA receptors
The addition of GABA (1 nmol/L–1 mmol/L) to membranes obtained from control and zolpidem pre-treated cells enhanced [3H]flunitrazepam binding in a concentration dependent manner. The data were introduced as a percentage of their own basal values and as a maximum enhancement (Emax) of [3H]flunitrazepam binding (Figure 6). The maximum enhancement of [3H]flunitrazepam binding (Emax) produced by GABA in the control group was 82.3%±3.7%, indicating that the GABA binding sites were functionally coupled to the benzodiazepine binding sites. The maximum enhancements of [3H]flunitrazepam binding in short-term zolpidem treated groups were not significantly different from the one produced in the control group (Emax values were: one-time, 2 h zolpidem treatment, 67.4%±7.8%; 2 h zolpidem treatment for two consecutive days, 67.6%±2.2%; and 2 h zolpidem treatment for three consecutive days, 72.55%±4.1%). The analysis of enhancement curves did not reveal differences in the concentrations of GABA that produced a half-maximum enhancement of [3H]flunitrazepam binding (EC50) in control and zolpidem treated groups.
The effect of short-term zolpidem (10 μmol/L for 2 h during one, two or three consecutive days) treatment on [3H]flunitrazepam binding to membranes from HEK 293 cells stably transfected with α1β2γ2s subunits of GABAA receptors. Data are expressed as percent of their own basal values (A) and as the maximum GABA-induced enhancements (B; Emax) of [3H]flunitrazepam binding. The points and bars are means±SEM. n=3–5. Statistical analysis showed no significant change among the groups (ANOVA followed by the Newman-Keuls test).
Discussion
Recent studies have provided evidence that benzodiazepines share the pharmacological profile of addictive drugs through cell-type specific expression of α1-containing GABAA receptors13,14,19. Non-benzodiazepine zolpidem acts selectively at α1 subunit-containing GABAA receptors and is considered to be devoid of addiction liability. To test whether zolpidem induces similar molecular changes as those reported to be linked to the development of tolerance, we conducted several experiments. The present results demonstrate that a single (2 h) or repeated (2 h per day for 2 or 3 d) exposure of stably transfected HEK 293 cells expressing recombinant α1β2γ2s GABAA receptors to hypnotic zolpidem did not affect the maximum number of [3H]flunitrazepam binding sites and their affinity. Stimulation studies revealed that the ability of GABA to potentate [3H]flunitrazepam binding in the control and short-term treated groups was not affected. In contrast, long-term (48 h) exposure of these cells to 1 or 10 μmol/L zolpidem enhanced the maximum number of benzodiazepine binding sites without changing their affinity. Long-term zolpidem occupation (48 h) of benzodiazepine binding sites at GABAA receptors produced a partial allosteric uncoupling of GABA and benzodiazepine binding sites, as evidenced by decreased ability of GABA to stimulate [3H]flunitrazepam binding.
It has been shown that a single intra-peritoneal injection of benzodiazepines is sufficient to induce synaptic plasticity in mice. It is postulated that observed early adaptive changes are not sufficient to explain long-term development of addiction. Instead, they represent an imperative initial step that triggers synaptic changes in addiction if the use of drug becomes chronic. Furthermore, it has been shown that benzodiazepine-induced changes in synaptic plasticity depend on α1-containing GABAA receptors because the observed changes are abolished in α1-H101R knock-in mice19. In our model, a single dose of zolpidem and short-term intermittent zolpidem treatment did not induce molecular changes at GABAA receptors regarding receptor number and GABA potentiation. This suggests that zolpidem might have a lower propensity for inducing molecular changes at GABAA receptors, possibly associated with the development of tolerance if used in a strict daily regime. On the other hand, as shown recently, long-term continuous occupation of GABAA receptors with zolpidem can induce adaptive changes at GABAA receptors13. It should be mentioned that these changes are not substantially different from those obtained after prolonged exposure of these cells to high doses of classical benzodiazepine-diazepam14,20. Moreover, Vlainić et al (2010)13 assumed that prolonged zolpidem treatment induces an increase of cell-surface GABAA receptors that are functionally active. Several potential mechanisms could underlie the up-regulation of GABAA receptor number: an increased synthesis, a decreased degradation of receptor proteins or an enhanced rate of receptor incorporation into membranes. The same authors also showed an increased level of α1 subunit mRNA and γ2 subunit proteins suggesting at least a partial role of transcriptional mechanisms in zolpidem-induced enhancement of GABAA receptors. Although mRNA changes do not necessarily reflect changes in protein expression21,22, Uusi-Oukari et al (2000)1 showed that there is tight control between the expression of α1 subunit mRNA and polypeptide. Moreover, it has been demonstrated that benzodiazepines regulate α123 and γ224 subunit mRNA at the level of transcription. A general trophic effect of zolpidem treatment on the growth of HEK 293 cells could presumably be excluded because total cellular proteins did not vary between the control and zolpidem-pre-treated group (data not shown).
Furthermore, 2-d zolpidem treatment produced functional uncoupling between the GABA and benzodiazepine binding sites, as demonstrated by the study of Primus et al (1996)25. The functional consequences of zolpidem-induced augmentation of GABAA receptor number observed in the study along with the reduced functional coupling were not determined. The exact molecular mechanism(s) leading to functional uncoupling between GABA and benzodiazepine binding sites remain unknown. Although uncoupling of the benzodiazepine and GABA binding sites could be produced by drugs inhibiting protein kinase A, it is supposed that direct phosphorylation of GABAA receptors is not involved in coupling/uncoupling processes26. The same authors proposed that prolonged benzodiazepine treatment induces internalization of surface GABAA receptors into intracellular vesicles, where the potentiation by GABA is impaired but the normal benzodiazepine binding can occur. However, several studies20,25 have failed to support the internalization model of GABAA receptors. The observed reduction in functional coupling between GABA and benzodiazepine binding sites could represent a conformational change at the receptor binding sites. It has been suggested that residues in and surrounding benzodiazepine binding site are aligned with the residues that form the GABA binding site27. Morlock and Czajkowski (2011)28 speculated that the positioning of the drug at the benzodiazepine binding site and/or the positioning of nearby residues induces different downstream allosteric rearrangements. Thus, allosteric uncoupling between GABA and benzodiazepine binding sites leads to a reduced potency of benzodiazepines. One cannot conclude that zolpidem-mediated activity in animals and humans will be reduced because prolonged zolpidem treatment produced an increase in GABAA receptor number. However, long-term administration of non-selective full positive allosteric modulator of GABA action at GABAA receptors leads to alterations in receptor expression and/or function, resulting in the development of tolerance and dependence. Many authors working either on animals29, neuronal cultures30,31,32 or recombinant receptors13,14,20,25,26,33,34 have found reduced allosteric linkage between GABA and benzodiazepine binding sites as a result of prolonged benzodiazepine action. Moreover, animals and humans treated for prolonged period of time with drugs acting as full positive modulators of GABA action at GABAA receptors developed tolerance characterized by a decreased ability of the drug to produce its pharmacological effect. Although it appears that allosteric uncoupling could explain the development of tolerance, the molecular mechanisms are rather more complex15,17.
In conclusion, it should be mentioned that zolpidem, which is highly selective for α1 subunit of GABAA receptors and is claimed to carry a low risk for addiction during long-term treatment, induces adaptive changes that are rather similar to those produced by long-term benzodiazepine treatment. Previous studies on mice10 and rats11 suggested that, upon repeated treatment, zolpidem produced tolerance to its anticonvulsive and sedative effects. Therefore, zolpidem has a higher abuse potential than previously suggested12. Since 2002, the World Health Organization has considered the frequency of zolpidem abuse and dependence to be similar to that of benzodiazepines.
Our results on intermittent short-term exposure suggest that, if used in a strong daily regime, zolpidem does not produce changes at recombinant GABAA receptors stably expressed in HEK 293 cells. This could presumably be associated with the development of tolerance, as it is with the continuous treatment13,35. The observed changes are not substantially different from those obtained after prolonged exposure of cells to high doses of classical benzodiazepines14.
Author contribution
Josipa VLAINIC and Danka PERIČIĆ conceived and designed the experiments. Maja Jazvinšćak JEMBREK and Josipa VLAINIC performed the experiments. Toni VLAINIĆ analyzed the data. Josipa VLAINIC and Toni VLAINIĆ wrote the paper. Dubravka Švob ŠTRAC helped with linguistic formulation of the text.
References
Uusi-Oukari M, Heikkilä J, Sinkkonen ST, Mäkelä R, Hauer B, Homanics GE, et al. Long-range interactions in neuronal gene expression: evidence from gene targeting in the GABAA receptor β2-α6-α1-γ2 subunit gene cluster. Mol Cell Neurosci 2000; 16: 34–41.
Olsen RW, Sieghart W . International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid (A) receptors: classification on the basis of subunit composition, pharmacology, and function. Pharmacol Rev 2008; 60: 243–60.
Arbilla S, Depoortere H, George P, Langer SZ . Pharmacological profile of the imidazopyridine zolpidem at benzodiazepine receptors and electrocorticogram in rats. Naunyn Schmiedebergs Arch Pharmacol 1985; 330: 248–51.
Depoortere H, Zivkovic B, Lloyd KG, Sanger DJ, Perrault G, Langer SZ, et al. Zolpidem, a novel nonbenzodiazepine hypnotic. I. Neuropharmacological and behavioral effects. J Pharmacol Exp Ther 1986; 237: 649–58.
Sanna E, Busonero F, Talani G, Carta M, Massa F, Peis M, et al. Comparison of the effects of zaleplon, zolpidem, and triazolam at various GABA(A) receptor subtypes. Eur J Pharmacol 2002; 451: 103–10.
Ci SQ, Ren TR, Ma CX, Su ZG . Modeling of alphak/gamma2 (k=1, 2, 3 and 5) interface of GABAA receptor and docking studies with zolpidem: implications for selectivity. J Mol Graph Model 2007; 26: 537–45.
Peričić D, Vlainić J, Švob Štrac D . Sedative and anticonvulsant effects of zolpidem in adult and aged mice. J Neural Transm 2008; 115: 795–802.
Vlainić J, Peričić D . Zolpidem is a potent anticonvulsant in adult and aged mice. Brain Res 2010; 1310: 181–8.
Zhang LL, Chen L, Xue Y, Yung WH . Modulation of synaptic GABAA receptor function by zolpidem in substantia nigra pars reticulata. Acta Pharmacol Sin 2008; 29: 161–8.
Vlainić J, Peričić D . Effect of acute and repeated zolpidem treatment on pentylenetetrazole-induced seizure threshold and on locomotor activity: comparison with diazepam. Neuropharmacol 2009; 56: 1124–30.
Auta J, Impagnatiello F, Kadriu B, Guidotti A, Costa E . Imidazenil: a low efficacy agonist at alpha1- but high efficacy at alpha5-GABAA receptors fail to show anticonvulsant cross tolerance to diazepam or zolpidem. Neuropharmacol 2008; 55: 148–53.
Victorri-Vigneau C, Dailly E, Veyrac G, Jolliet P . Evidence of zolpidem abuse and dependence: results of the French Centre for Evaluation and Information on Pharmacodependence (CEIP) network survey. Br J Clin Pharmacol 2007; 64: 198–209.
Vlainić J, Jazvinšćak Jembrek M, Švob Štrac D, Peričić D . The effects of zolpidem treatment and withdrawal on the in vitro expression of recombinant α1β2γ2s GABAA receptors expressed in HEK 293 cells. Naunyn Schmiedebergs Arch Pharmacol 2010; 382: 201–12.
Švob Štrac D, Vlainić J, Jazvinšćak Jembrek M, Peričić D . Differential effects of diazepam treatment and withdrawal on recombinant GABAA receptor expression and functional coupling. Brain Res 2008; 1246: 29–40.
Bateson AN . Basic pharmacological mechanisms involved in benzodiazepine tolerance and withdrawal. Curr Pharm Des 2002; 8: 5–21.
Uusi-Oukari M, Korpi ER . Regulation of GABAA receptor subunit expression by pharmacological agents. Pharmacol Rev 2010; 62: 97–135.
Tan KR, Rudolph U, Lüscher C . Hooked on benzodiazepines: GABAA receptor subtypes and addiction. Trends Neurosci 2011; 34: 188–97.
Peričić D, Jazvinšćak Jembrek M, Švob Štrac D, Lazić J, Špoljarić IR . Enhancement of benzodiazepine binding sites following chronic treatment with flumazenil. Eur J Pharmacol 2005; 507: 7–13.
Tan KR, Brown M, Labouèbe G, Yvon C, Creton C, Fritschy JM, et al. Neural bases for addictive properties of benzodiazepines. Nature 2010; 463: 769–74.
Peričić D, Švob Štrac D, Jazvinšćak Jembrek M, Vlainić J . Allosteric uncoupling and up-regulation of benzodiazepine and GABA recognition sites following chronic diazepam treatment of HEK 293 cells stably transfected with alpha1beta2gamma2S subunits of GABA(A) receptors. Naunyn Schmiedebergs Arch Pharmacol 2007; 375: 177–87.
Impagnatiello F, Pesold C, Longone P, Caruncho H, Fritschy JM, Costa E, et al. Modifications of γ-aminobutyric acid A receptor subunit expression in rat neocortex during tolerance to diazepam. Mol Pharmacol 1996; 49: 822–31.
Pesold C, Caruncho HJ, Impagnatiello F, Berg MJ, Fritschy JM, Guidotti A, et al. Tolerance to diazepam and changes at GABAA receptor subunit expression in rat neocortical areas. Neuroscience 1997; 79: 477–87.
Kang I, Lindquist DG, Kinane B, Ercolani L, Pritchard GA, Miller LG . Isolation and characterization of the promoter of the human GABAA receptor α1 subunit gene. J Neurochem 1994; 62: 1643–6.
Holt RA, Bateson AN, Martin IL . Chronic zolpidem treatment alters GABA(A) receptor mRNA levels in the rat cortex. Eur J Pharmacol 1997; 329: 129–32.
Primus RJ, Yu J, Xu J, Hartnett C, Meyyappan M, Kostas C, et al. Allosteric uncoupling after chronic benzodiazepine exposure of recombinant γ-aminobutyric acid A receptors expressed in Sf9 cells: ligand efficacy and subtype selectivity. J Pharmacol Exp Ther 1996; 276: 882–90.
Ali NJ, Olsen RW . Chronic benzodiazepine treatment of cells expressing recombinant GABA(A) receptors uncouples allosteric binding: studies on possible mechanisms. J Neurochem 2001; 79: 1100–8.
Williams DB, Akabas MH . Evidence for distinct conformations of the two alpha1 subunits in diazepam-bound GABA(A) receptors. Neuropharmacol 2001; 41: 539–45.
Morlock EV, Czajkowski C . Different residues in the GABAA receptor benzodiazepine binding pocket mediate benzodiazepine efficacy and binding. Mol Pharmacol 2011; 80: 14–22.
Gallager DW, Lakoski JM, Gonsalves SF, Rauch SL . Chronic benzodiazepine treatment decreases postsynaptic GABA sensitivity. Nature 1984; 308: 74–7.
Roca DJ, Schiller GD, Friedman L, Rozenberg I, Gibbs TT, Farb DH . γ-Aminobutyric acid A receptor regulation in culture: altered allosteric interactions following prolonged exposure to benzodiazepines, barbiturates, and methylxanthines. Mol Pharmacol 1990; 37: 710–9.
Hu XJ, Ticku MK . Chronic benzodiazepine agonist treatment reduces functional uncoupling of the γ-aminobutyric acid-benzodiazepine receptor ionophore complex in cortical neurons. Mol Pharmacol 1994; 45: 618–25.
Friedman LK, Gibbs TT, Farb DH . Gamma-aminobutyric acidA receptor regulation: heterologous uncoupling of modulatory site interactions induced by chronic steroid, barbiturate, benzodiazepine, or GABA treatment in culture. Brain Res 1996; 707: 100–9.
Klein RL, Whiting PJ, Harris RA . Benzodiazepine treatment causes uncoupling of recombinant GABAA receptors expressed in stably transfected cells. J Neurochem 1994; 63: 2349–52.
Klein RL, Mascia MP, Harkness PC, Hadingham KL, Whiting PJ, Harris RA . Regulation of allosteric coupling and function of stably expressed γ-aminobutyric acid (GABA)A receptors by chronic treatment with GABAA and benzodiazepine agonists. J Pharmacol Exp Ther 1995; 274: 1484–92.
Vlainić J, Strac DŠ, Jembrek MJ, Vlainić T, Peričić D . The effects of zolpidem treatment on GABA(A) receptors in cultured cerebellar granule cells: changes in functional coupling. Life Sci 2012; 90: 889–94.
Acknowledgements
The study was supported by the Croatian Ministry of Science, Education and Sports (Project: Stress, GABAA receptors and mechanisms of action of neuro-psychoactive drugs). The skillful technical assistance of Mrs Zlatica TONŠETIĆ is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vlainić, J., Jembrek, M., Vlainić, T. et al. Differential effects of short- and long-term zolpidem treatment on recombinant α1β2γ2s subtype of GABAA receptors in vitro. Acta Pharmacol Sin 33, 1469–1476 (2012). https://doi.org/10.1038/aps.2012.89
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2012.89