Abstract
Aim:
To establish a population pharmacokinetics (PPK) model of levetiracetam in Chinese children with epilepsy.
Methods:
A total of 418 samples from 361 epileptic children in Peking University First Hospital were analyzed. These patients were divided into two groups: the PPK model group (n=311) and the PPK validation group (n=50). Levetiracetam concentrations were determined by HPLC. The PPK model of levetiracetam was established using NONMEM, according to a one-compartment model with first-order absorption and elimination. To validate the model, the mean prediction error (MPE), mean squared prediction error (MSPE), root mean-squared prediction error (RMSPE), weight residues (WRES), and the 95% confidence intervals (95% CI) were calculated.
Results:
A regression equation of the basic model of levetiracetam was obtained, with clearance (CL/F)=0.988 L/h, volume of distribution (V/F)=12.3 L, and Ka=1.95 h−1. The final model was as follows: Ka=1.56 h−1, V/F=12.1 (L), CL/F=1.04×(WEIG/25)0.583 (L/h). For the basic model, the MPE, MSPE, RMSPE, WRES, and the 95%CI were 9.834 (−0.587–197.720), 50.919 (0.012–1286.429), 1.680 (0.021–34.184), and 0.0621 (−1.100–1.980). For the final model, the MPE, MSPE, RMSPE, WRES, and the 95% CI were 0.199 (−0.369–0.563), 0.002082 (0.00001–0.01054), 0.0293 (0.001−0.110), and 0.153 (−0.030–1.950).
Conclusion:
A one-compartment model with first-order absorption adequately described the levetiracetam concentrations. Body weight was identified as a significant covariate for levetiracetam clearance in this study. This model will be valuable to facilitate individualized dosage regimens.
Similar content being viewed by others
Introduction
Levetiracetam (LEV; (S)-ethyl-2-oxo-pyrrolidine acetamide (Keppra®), UCB Pharma, Braine-l'Alleud, Belgium) is a new antiepileptic drug. It is mainly used for the adjunctive treatment of partial-onset seizures in adults and children, as well as myoclonic and primary generalized tonic-clonic seizures in patients with idiopathic generalized epilepsy1,2,3. The primary mechanism of action of LEV relates to its binding to synaptic vesicle proteins4. The results of clinical trials in a Chinese population have demonstrated that LEV is effective and well tolerated in adults with inadequately controlled partial-onset seizures5.
LEV shows linear pharmacokinetics, and its major route of elimination is through the kidneys, with approximately 66% of a dose eliminated unchanged and 27% as inactive metabolites6,7,8,9. Renal function determines the rate of elimination of LEV. The half-life is 6–8 h in healthy adults, and 5–7 h in children aged 6–12 years. However, the apparent clearance is 30%–40% higher in children than in adults10. The initial daily dose is 20 mg−1·kg−1·d−1 (10 mg/kg twice daily) and can go up to 60 mg−1·kg−1·d−1. Even higher doses (>60 mg−1·kg−1·d−1) have also been reported11.
Measuring the serum concentration (SC) of LEV can be useful in assessing compliance and managing patients in situations associated with pharmacokinetic (PK) alterations in pathological states such as renal impairment, as well as in specific age groups such as children and the elderly12,13. Numerous chromatographic methods for the quantification of LEV in serum have been described. These include high performance liquid chromatography (HPLC) with ultraviolet (UV) detection and gas chromatography (GC) with various detection systems14,15,16.
Levetiracetam has been used in the treatment of children with epilepsy in China since 2007; however, the PK parameters in Chinese children are not known. Therefore, the aims of the present study were to develop a population pharmacokinetics (PPK) model of levetiracetam in Chinese children with epilepsy.
Materials and methods
Patients
Children with epilepsy aged 0.5–14 years were recruited by pediatricians at outpatient clinics in Peking University First Hospital. They were treated with LEV monotherapy or adjunctive therapy for 1 week at least on a stable LEV dose treatment. They presented with various types of epilepsy syndromes, including partial, generalized and undetermined. For each patient, the time between dosing and sampling, gender, age, weight, serum concentration, and co-administered medications were recorded. LEV tablets were administered in daily doses of 20–60 mg/kg. The LEV dose regimen could be adjusted in cases of inadequate seizure control or side effects.
Sample collection
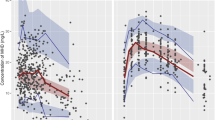
The sampling times to last LEV intake were generally between 1 h and 13 h (Figure 1). Blood samples were kept at room temperature for 30 min before they were centrifuged, and the separated serum was preserved at -20 °C in our laboratory for less than a week before analysis.
Analytical method
This method has already been successfully established in China17. Briefly, the HPLC system consists of a Waters 1525 (Waters company), including a manual sampler, a degasser, a quaternary pump, a thermostatted column compartment and a variable wavelength detector. The chromatographic separation of the analyte is done on an Alltima C18 (Grace Davison Discovery Sciences Company, Deerfield, IL, USA, 150 mm×4.6 mm, 5 mm particle size) analytical column protected with a pre-filter. Data were collected and analyzed using a Breeze software package, version 3.03. LEV and an internal standard UCB17025 were provided by UCB Pharma (Brain-l'Alleud, Belgium). By spiking drug-free human serum with a working solution, calibration samples between 160 and 1.25 mg/L were obtained. A linear regression was performed from chromatographic data that allowed us to extrapolate the LEV concentration in each patient sample. Quality control samples were prepared at three concentration levels, with target values of 2, 16, and 80 mg/mL. An internal standard (100 μL of a 40 μg/mL solution) and 1 mL dichloromethane were added to 100 μL serum sample. After vortexing for 1 min, ultrasonication for 10 min and centrifuging at 4000×g for 10 min, the upper layer was removed and evaporated to dryness under a nitrogen stream at room temperature. The residue was reconstituted in 100 μL methanol, of which 20 μL was injected into the chromatographic system. The flow rate was 1 mL/min and the column temperature was 37 °C. The wavelength detection was set at 210 nm. The retention time of LEV and UCB17025 was 5.45±0.10 and 7.50±0.20 min under the described conditions, respectively.
PPK modeling
After parameterization according to previous studies11,18, the LEV concentrations were suited to using a one-compartment model and a first-order absorption process. The PPK modeling included the base model and final model.
PPK model of LEV
The PK data were analyzed with the use of nonlinear mixed effects modeling (NONMEM, version 7, level 1.2). To describe the PK of LEV, the PK disposition model was tested using a standard one-compartment model with subroutine ADVAN2 TRANS2. The first order conditional estimation (FOCE) was used to develop the model. Firstly, the basic model with inter-individual variability was set up. The model was parameterized for apparent clearance (CL/F), the apparent volumes of distribution of the central compartment (V/F), and the absorption rate constant (Ka). CL/F=θCL/F·exp(ηCL/F); V/F=θV/F·exp(ηV/F); Ka=θKa·exp(ηKa). Secondly, the covariate variability was added step by step and the full PPK model was set as follows:

where Pi is the individual predicted parameter value, θi is the typical population estimate of Pi, ηi is the proportional difference, COV and mCOV are the individual and median covariate values, and θj is the power factor for the effect of the covariate on Pi. The effects of categorical covariates on the structural parameters were modeled as follows:

where Pj is the individual predicted parameter value, θ1j is the typical population value of Pj for category 1 of the covariate, and N1j is an indicator variable that has a value of 1 when the covariate is present and 0 when the covariate is absent. The covariates of this study included: age (year), weight (WEIG), dose [the dosage of whole day (mg) before sampling] and co-administered medications (CO)].
Data analysis
When the important covariates were selected, a stepwise forward and backward approach was used and each covariate was added or deleted individually. Sex and CO were the categorical covariates. These categorical covariates were modeled by the use of indicator variables. The influences of continuous covariates, such as age, weight, dosage, were also explored. The likelihood ratio test was used to determine the appropriateness of a selected covariate. A decrease in the objective function values (OFV) (−2 log-likelihood) of 7.88 units was considered significant (χ2P<0.005, df=1). Throughout the process of model development, graphic methods were also used to judge the general goodness of fit.
Statistical model
When an influence of the fixed effect was not considered, individual PK parameters were typical population values plus the random deviation. According to the experiential formulations, inter-individual and intra-individual deviations (residual deviation) were presented as follows:

where Pj is the jth patient PK parameter; PTV is the typical value of P for the population, ηp is inter-individual deviation (a mean of 0 and variance ωP), Eij0 is the observation value, Eij is the prediction value of Eij0, and εij is the intra-individual deviation(its mean is 0 and variance is σE2).
Model validation
To validate the basic and the final model, concentrations from 50 patients in the valid group were predicted by the two models. These patients were enrolled at random. To assess the accuracy and precision of the concentration prediction, the mean prediction error (MPE), mean squared prediction error (MSPE), root mean-squared prediction error (RMSPE), weight residues (WRES), and the 95% confidence intervals (95% CI) were calculated. Then, the values of the two models were compared19. The shrinkage for each of the parameters in the model was evaluated using the method described by Karlsson20.
Results
Patient demographics
A total of 418 samples obtained from 361 patients aged from 0.5–14 years were available for PK modeling. The characteristics of the studied population are summarized in Table 1. The intervals between the last dosage time and sampling time were distributed over 1–13 h (Figure 1). The distributions of the intervals between the last dosage time and sampling time in the model group are shown in the Figure 2. All of the patients had normal renal and hepatic function. In this population, 40% and 60% used one or two concomitant anti-epileptic drugs (AEDs), respectively. The most frequently used concomitant AEDs were valproic acid (VPA), lamotrigine (LTG), carbamazepine (CBZ), oxcarbazepine (OXC), and topiramate (TPM).
PPK modeling
A classical one-compartment model with first-order absorption, and linear elimination (ADVAN2 and TRANS2) best described the data. The distributions of concentration data and sampling times in the PPK model and PPK valid groups are shown in Figure 1. In the basic model, Ka=1.95 (h−1), V/F=12.3 (L), and CL/F=0.988 (L/h). In the full regressive model, the results of all the covariates were validated by a hypothesis test (Table 2). The parameters of the final model are shown in Table 3, and the final model was as follows: Ka=1.56 (h−1), V/F=12.1 (L), CL/F=1.04×(WEIG/25)0.583 (L/h). No significant interaction with the concomitant AEDs was found. The estimated levetiracetam CL/F was 1.04 L/h and the corresponding half-life estimate in these subpopulations was 8.13 h.
Model validation
Comparison between scattergrams of basic and final model
Diagnostic plots are shown in Figure 3, including Dependent Variable (DV) versus Prediction (PRED), DV versus Individual Prediction (IPRED), weighted residual error (CWRES) versus PRED, and CWRES versus TIME.
Diagnostic plots of the final PK model. (A) Plot of the observed concentrations versus population predicted levetiracetam concentrations (PRED). B) Plot of the observed concentrations versus individual population predicted levetiracetam concentrations (IPRED). (C) Plot of population predicted levetiracetam concentrations (PRED) versus CWRES. (D) Plot of CWRES versus TIME. DV: Dependent Variable.
Comparison of prediction errors between the final model and basic model
MPE, MSPE, RMSPE, WRES and 95% CI in the basic model and final model are defined in Table 3. The indicators in the final model, such as MPE, MSPE, and RMSPE, decreased and showed more accurate predictions. The shrinkage for each of the parameters in the model is shown in Table 4.
Discussion
In this study, a one-compartment model with first-order absorption and elimination best characterized the data. The model describes the data adequately. The mean CL/F, V/F, and Ka were 1.04 L/h (0.69 mL·min−1·kg−1), 12.1 L and 1.56 h−1, respectively. No drug-drug interaction was observed in this study. In this model, the median WEIG in our population was 25 kg. Weight was identified as the most important covariate that explained the inter-individual variability of the apparent serum clearance of LEV.
Patient data
There are very sparse PK samples for modeling. There were not enough points in the absorption phase (Figures 1 & 2); therefore, the ωKa was fixed at 0. There was insufficient information about the absorption and distribution phases, which may have resulted in potential bias and imprecision regarding the parameter estimates. More attention should be paid to the distribution of the blood sampling time.
Comparison with similar domestic research
Zhao et al21 studied healthy Chinese male subjects following a single-dose of either 500 mg and 1500 mg of levetiracetam, the median tmax was 0.5 h; t1/2 was 7.3±0.8 and 7.3±0.7 h. The pharmacokinetic data obtained in these Chinese subjects were similar to the historical data from a matched group of white subjects. There are no related studies of PPK of LEV in Chinese adults with epilepsy.
Comparison with similar overseas studies
Pigeole et al22 found the following parameters in Japanese and Western adults: Ka(h−1)=2.44 (fed intake) or 4.80 (fasted intake), L/F(L/h)=4.02*(WT/70)0.268*(CLCR/110)0.122*S*M, F(L)=52.7*(WT/70)0.952*P*VA, where WT is the bodyweight in kg; CLCR is creatinine clearance in mL/min; S=1 for males and 0.896 for females; M=1.09 for enzyme-inducing AEDs, 0.812 for valproic acid and 1 for other AEDs; P=1 for epileptic subjects and 0.861 for healthy subjects, and VA=0.776 for valproic acid and 1 for other AEDs. Glauser et al23 found that CL/F was 1.46±0.42 mL·min−1·kg−1 in patients aged from 2.3 to 46.2 months. Toublanc et al17 found in children aged between 3 months and 18 years, CL/F (L/h)=2.18*K*(WEIG/30)0.753, K=1 for children not receiving enzyme-inducing AEDs and K=1.22 in the presence of enzyme-inducing AEDs. K corresponds to the typical fold increase in LEV clearance by enzyme inducers. V/F (L/h)=21.4*(WEIG/30)0.898, Ka(h−1)=1.48*(Age/10)0.277. Chhun et al18 found that, from 4 to 16 years, CL/F (L/h)= 2.47*(BW/33)0.89, V/F (L)=21.9*(BW/33)0.93, and Ka (h−1)=3.83. The CL/F of this study was lower than in the children in the studies by Toublanc and Chhun17,18. In Merhar's study of neonates24, clearance was 1.21 mL·min−1·kg−1. In Pellock's study12 of 6–12 years old, CL/F was 1.43 mL·min−1·kg−1, which was higher than that in adults (0.96 mL·min−1·kg−1) and than the 0.69 mL·min−1·kg−1 observed in the current study. It appears that the CL/F of Chinese children is lower than that of white Caucasian children. The trough serum concentration was also higher than in the white children (Table 5).
It appears that the CL/F in Chinese children was approximately 50% lower than in Western children based on the published data (eg, 0.69 mL·min−1·kg−1 vs 1.21–1.46 mL·min−1·kg−1). LEV was mainly eliminated by the kidneys, and significant ethnic differences were not expected in previous studies; however, racial differences are likely to be at least partly responsible for the difference in CL/F that we observed, and these differences will be the subject of future studies. Our study has a good representation of Chinese children with epilepsy with ages that ranged from 0.5 to 14 years.
Validation of the PPK model
The final model contained covariates, such as age, weight, concomitant medication, and different formulations, and it was more accurate in predicting the patients' blood concentrations than the basic model that had no covariates. For the basic model, the MPE, MSPE, RMSPE, WRES, and their 95% CIs were 9.834 (−0.587–197.720), 50.919 (0.012–1286.429), 1.680 (0.021–34.184), and 0.0621 (−1.100–1.980), respectively. For the final model, the MPE, MSPE, RMSPE, WRES, and their 95% CIs were 0.199 (−0.369–0.563), 0.002082 (0.00001–0.01054), 0.0293 (0.001–0.110), and 0.153 (−0.030–1.950). Furthermore, the RMSPE (95% CI) of 0.0293 (0.001–0.110) in the final model outweighed the basic model's value of 1.680 (0.021–34.184). Therefore, the final model had a better accuracy and precision than the basic model. The shrinkage values of ωCL/F, ωV/F and ε were 20%, 44.9%, and 31%, respectively. The shrinkage of ωV/F (44.9%) was caused by insufficient information regarding the distribution phase (Figure 2). Based on this final PPK model, individual PK parameters will be estimated by the Bayesian approach in the near future, which will facilitate individualized dosage regimens.
Conclusion
The population analysis has been successful in describing the pharmacokinetics of LEV in children aged 0.5–14 years. A one-compartment model with first-order absorption adequately described the LEV concentrations. The findings indicate that weight was the most influential factor for the CL/F of LEV in children with normal renal function. This will be invaluable for the development of individualized dosage regimens.
Author contribution
Li WANG designed research; Ye WU and Min-ji WEI performed research; Wei LU and De-wei SHANG contributed new analytical tools and reagents; De-wei SHANG and Ying-hui WANG analyzed data; Ying-hui WANG wrote the paper.
References
Stefan H, Feuerstein TJ . Novel anticonvulsant drugs. Pharmacol Ther 2007; 113: 165–83.
Shorvon SD, Lowenthal A, Janz D . Multicenter double-blind, randomized, placebo-controlled trial of levetiracetam as add-on therapy in patients with refractory partial seizures. European Levetiracetam Study Group. Epilepsia 2000; 41: 1179–86.
Hwang H, Kim KJ . New antiepileptic drugs in pediatric epilepsy. Brain Dec 2008; 30: 549–55.
Stockist A, Lu S, Tonner F . Clinical pharmacology of levetiracetam for the treatment of epilepsy. Expert Rev Pharmacol 2009; 2: 339–50.
Zhou B, Zhang Q, Tian L, Xiao J, Stefan H, Zhou D . Effects of levetiracetam as an add-on therapy on cognitive function and quality of life in patients with refractory partial seizures. Epilepsy Behav 2008; 12: 305–10.
Patsalos PN . Clinical pharmacokinetics of levetiracetam. Clin Pharmacokinet 2004; 43: 707–24.
Fay MA, Sheth RD, Gidal BE . Oral absorption kinetics of levetiracetam: the effect of mixing with food or enteral nutrition formulas. Clin Ther 2005; 27: 594–8.
Patsalos PN, Ghattaura S, Ratnaraj N, Sander JW . In situ metabolism of levetiracetam in blood of patients with epilepsy. Epilepsia 2006; 47: 1818–21.
Allegaert K, Lewi L, Naulaers G, Lagae L . Levetiracetam pharmacokinetics in neonates at birth. Epilepsia 2006; 47: 1068–9.
Glauser TA, Mitchell WG, Weinstock A, Bebin M, Chen D, Coupez R, et al. Pharmacokinetics of levetiracetam in infants and young children with epilepsy. Epilepsia 2007; 48: 1117–22.
Toublanc N, Sargentini-Maier ML, Lacroix B, Jacqmin P, Stockis A . Retrospective population pharmacokinetic analysis of levetiracetam in chidren and adolescents with epilepsy. Clin Pharmacokinet 2008; 47: 333–41.
Pellock JM, Glauser TA, Bebin EM, Fountain NB, Ritter FJ, Coupez RM, et al. Pharmacokinetic study of levetiracetam in children. Epilepsia 2001; 42: 1574–9.
Ensom MH, Chang TK, Patel P . Pharmacogenetics: the therapeutic drug monitoring of the future? Clin Pharmacokinet 2001; 40: 783–802.
Juenke J, Brown PI, Urry FM, McMillin GA . Drug monitoring and toxicology: a procedure for the monitoring of levetiracetam and zonisamide by HPLC-UV. J Anal Toxicol 2006; 30: 27–30.
Lancelin F, Franchon E, Kraoul L . Therapeutic drug monitoring of levetiracetam by high-performance liquid chromatography with photodiode array ultraviolet detection: preliminary observations on correlation between plasma concentration and clinical response in patients with refractory epilepsy. Ther Drug Monit 2007; 29: 576–83.
Contin M, Mohamed S, Albani F, Riva R, Baruzzi A . Simple and validated HPLC-UV analysis of levetiracetam in deproteinized plasma of patients with epilepsy. J Chromatogr B Analyt Technol Biomed Life Sci 2008; 873: 129–32.
Wang Y, Wei MJ, Wang YX, Wang L . Therapeutic drug monitoring of levetiracetam by HPLC-UV. J Pediatr Pharm 2010; 16: 34–7.
Chhun S, Jullien V, Rey E, Dulac O, Chiron C, Pons G . Population pharmacokinetics of levetiracetam and dosing recommendation in children with epilepsy. Epilepsia 2009; 50: 1150–7.
Jiang DC, Wang L, Wang YQ, Li L, Lu W, Bai XR . Population pharmacokinetics of valproate in Chinese children with epilepsy. Acta Pharmacol Sin 2007; 28: 1677–84.
Karlsson MO, Savic RM . Diagnosing model diagnostics. Clin Pharmacol Ther 2007; 82: 17–20.
Zhao Q, Jiang J, Li X, Lu ZS, Hu P . Epilepsy Therapy: Study data from Peking Union Medical College, Medical college update knowledge of epilepsy therapy. Pain Central Nerv Sys Week 2007: 5: 83.
Pigeolet E, Jacqmin P, Sargentini-Maier ML, Stockis A . Population pharmacokinetics of levetiracetam in Japanese and Western adult. Clin Pharmacokinet 2007; 46: 503–13.
Glauser TA, Mitchell WG, Weinstock A, Bebin M, Chen D, Coupez R, et al. Pharmacokinetics of levetiracetam in infants and young children with epilepsy. Epilepsia 2007; 48: 1117–22.
Merhar SL, Schibler KR, Sherwin CM, Meinzen-Derr J, Shi J, Balmakund T, et al. Pharmacokinetics of levetiracetam in neonates with seizures. J Pediatr 2011; 159: 152–4.
Fountain NB, Conry JA, Rodríguez-Leyva I, Gutierrez-Moctezuma J, Salas E, Coupez R, et al. Prospective assessment of levetiracetam pharmacokinetics during dose escalation in 4- to 12-year-old children with partial-onset seizures on concomitant carbamazepine or valproate. Epilepsy Res 2007; 74: 60–9.
Acknowledgements
This work was supported by grants from the UCB Pharma (Braine-l′Alleud, Belgium) and the Capital Development Fund of Medical Research of China (No 2009-2021). The authors thank the pediatricians at Peking University First Hospital (Beijing, China), for their contributions to the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Yh., Wang, L., Lu, W. et al. Population pharmacokinetics modeling of levetiracetam in Chinese children with epilepsy. Acta Pharmacol Sin 33, 845–851 (2012). https://doi.org/10.1038/aps.2012.57
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2012.57
Keywords
This article is cited by
-
Population Pharmacokinetics of Levetiracetam: A Systematic Review
Clinical Pharmacokinetics (2021)
-
Levetiracetam Clinical Pharmacokinetic Monitoring in Pediatric Patients with Epilepsy
Clinical Pharmacokinetics (2017)
-
Population pharmacokinetic models of lamotrigine in different age groups of Chinese children with epilepsy
European Journal of Clinical Pharmacology (2017)