Abstract
Aim:
E-cadherin is unusually highly expressed in most ovarian cancers. This study was designed to investigate the roles of E-cadherin in the carcinogenesis and progression of ovarian cancers.
Methods:
Human ovarian adenocarcinoma cell line SKOV-3 was examined. E-cadherin gene CDH1 in SKOV-3 cells was knocked down via RNA interference (RNAi), and the resultant variation of biological behavior was observed using CCK-8 and colony formation experiment. E-cadherin-mediated Ca2+-dependent cell-cell adhesion was used to study the mechanisms underlying the effects of E-cadherin on the proliferation and survival of SKOV-3 cells. The expression levels of E-cadherin, extracellular signal-related kinase (ERK), phosphorylated ERK (P-ERK) were measured using Western blot assays.
Results:
Transfection with CDH1-siRNA for 24–96 h significantly suppressed the growth and proliferation of SKOV-3 cells. E-cadherin-mediated calcium-dependent cell-cell adhesion of SKOV-3 cells resulted in a rapid increase of P-ERK, but did not modify the expression of ERK protein. The phosphorylation of ERK in the cells was blocked by pretreatment with the MEK1 specific inhibitor PD98059 (50 μmol/L), but not by the PI3K inhibitor wortmannin (1 μmol/L) or PKA inhibitor H89 (10 μmol/L).
Conclusion:
E-cadherin may function as a tumor proliferation enhancer via activating the MEK/ERK pathway in development of ovarian epithelial cancers.
Similar content being viewed by others
Introduction
Cadherins are a family of cell surface glycoproteins that mediate calcium-dependent intercellular adhesion, and are involved in cell differentiation and morphogenesis1, 2. E-cadherin is a 120 kDa transmembrane protein, encoded by the CDH1 gene at 16q22.1. E-cadherin can not only help establish calcium-dependent cell-cell contact through its extracellular domain, but also link the extracellular environment to the contractile cytoskeleton inside cells by the interaction of its short intracellular tail with catenins, which in turn bind to actin filaments and play an important role in certain nuclear responses3, 4, 5.
E-cadherin has been generally regarded as a tumor suppressor in various malignancies, such as lung, gastric, laryngeal, pancreatic, and bladder cancers. This is based on the fact that E-cadherin can prevent tumorigenesis, invasion, and metastasis via promoting cell-cell adhesions and inhibiting epithelial-mesenchymal transition (EMT)6, 7, 8, 9, 10, 11. However, a growing body of studies has recently emerged to challenge this view. For instance, E-cadherin is reported to be necessary for anchorage-independent growth and suppression of apoptosis in oral squamous cancer cells12. Expression of the E-cadherin-catenin cell adhesion complex is shown to be vital for disease progression in primary squamous cell carcinomas of the head and neck and their nodal metastases13. Most importantly, E-cadherin appears to function differently in the development of ovarian cancers. E-cadherin is not expressed by the normal human ovarian surface epithelium (OSE), whereas it can be detected in the OSE located in the deep clefts, inclusion cysts, and invaginations14, where over 90% of the ovarian cancers arise15. E-cadherin expression has been found in malignant ovarian tumors of all stages and its level is significantly higher in ovarian cancer tissues than in normal ovarian tissues14, 16, 17. Furthermore, when introduced into OSE, E-cadherin stimulates the secretion of the ovarian cancer-associated marker CA125 and the anchorage-independent growth in vitro and induces the growth, invasion, and metastasis of adenocarcinoma18, 19.
The above results suggest that the up-regulation of E-cadherin may be an early event in the initial development of ovarian epithelial cancers, which is opposite to its hypothesized role as a tumor suppressor. However, the mechanism that how E-cadherin plays its role during the development of ovarian cancer still remains unclear. Moreover, contradictory data have suggested that E-cadherin could inhibit tumor cell growth by suppressing phosphatidylinositol 3-kinase (PI3K)/Akt signaling in ovarian cancer cells20. In the present study, to determine the possible functions of E-cadherin in ovarian cancer cells, we knocked down the CDH1 gene expression via RNA interference (RNAi) in the SKOV-3 ovarian cancer cells. We also established an E-cadherin-mediated calcium-dependent cell-cell adhesion model by a method described before21, to detect its role and related signaling mechanism in the regulation of ovarian cancer cell growth and proliferation.
Materials and methods
Cell culture and reagents
The human ovarian adenocarcinoma cell line, SKOV-3, was kept in our laboratory and maintained in RPMI-1640 (Gibco, Paisley, UK) supplemented with 10% fetal calf serum (Gibco) at 37 °C in a 5% CO2 humidified atmosphere. To establish the calcium-dependent cell-cell adhesion model, SKOV-3 cells were serum starved overnight, and then were suspended in serum-free medium containing 4 mmol/L EGTA for 30 min to disrupt the calcium-dependent and E-cadherin-mediated cell-cell contacts. Thereafter, intercellular interactions were allowed to reestablish while the cells were re-suspended in fresh CaCl2 solution (final concentration of CaCl2, 1.8 mmol/L) for different periods ranging from five to thirty minutes. To block the function of certain kinases in signaling transduction experiments, cells were pretreated with kinase inhibitors before EGTA treatment. The SKOV-3 cells transfected by plasmids including siRNA targeting E-cadherin were treated in the same way as above 48 h after transfection.
The MEK1 inhibitor, PD98059, was purchased from Cell Signaling Technologies (Danvers, MA, USA). The PKA inhibitor, H89, was from Upstate Biotechnology (Boston, MA, USA). The PI3K inhibitor, wortmannin, was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The mouse anti-human E-cadherin monoclonal antibody and mouse monoclonal antibody to β-actin were purchased from BD Transduction Laboratories (SanJose, CA, USA). Neutralizing antibody to E-cadherin, SHE 78-7, was from Takara (Shiga, Japan). Rabbit polyclonal antibodies to p44/42 ERK, and phosphorylated p44/42 ERK (Thr202/Tyr204), were obtained from Cell Signaling Technologies (Beverly, MA, USA). Rabbit anti-mouse IGG-FITC secondary antibodies were from Zymed Laboratories, Inc (South San Francisco, CA, USA). Lipofectamine 2000 for transfection of small interfering RNA (siRNA) oligonucleotides was obtained from Invitrogen (Carlsbad, CA, USA).
RNA interference (RNAi)
SKOV-3 cells were seeded in 24-well plates 24 h before transfection at a density of 3×104 cells/well. Plasmids (1 μg per well) including siRNA targeting E-cadherin were mixed with Lipofectamin 2000 for transfection according to the manufacturer's instructions. The sequence of the double-stranded E-cadherin-specific siRNA was 5′-GGCCTCTACGGTTTCATAA-3′. The E-cadherin siRNA expressing plasmid and the negative control plasmid HK were purchased from Wuhan Gensil Biotechnology (Wuhan, China) and used directly for transfection.
Immunofluorescence staining
SKOV-3 cells cultured on glass coverslips were washed with PBS, fixed in 4% paraformaldehyde for 20 min at room temperature, and permeabilized with 0.5% Triton X-100 for 10 min at room temperature. The cells were then washed with PBS, sequentially stained with mouse anti-E-cadherin monoclonal antibody (1:100) for 1 h followed by fluorescent isothiocyanate (FITC)-labeled anti-mouse IgG antibody (1:50) (Zymed Laboratories, San Francisco, CA, USA) for 30 min at 37 oC, and mounted for visualization under a Zeiss Axiophot microscope (Carl Zeiss, Thornwood, NY, USA).
Western blot
To test the possible role of E-cadherin, MEK, PI3K and PKA, cells were pretreated with corresponding protein kinase inhibitors such as E-cadherin-specific neutralizing antibody SHE 78-7 (20 μg/mL), MEK1 specific inhibitor PD98059 (50 μmol/L), PI3K inhibitor wortmannin (1 μmol/L) or PKA inhibitor H89 (10 μmol/L) respectively for 30 min before being restored with fresh calcium for 10 min. At different time points after calcium restoration, certain cells were harvested and lysed in TNES buffer: 50 mmol/L Tris-HCl (pH 7.5), 2 mmol/L EDTA, 100 mmol/L NaCl, 1.0 mmol/L sodium orthovanadate, and 1% NP40 containing protease inhibitors (20 μg/mL aprotinin, 20 μg/mL leupeptin, and 1.0 mmol/L phenylmethylsulfonyl fluoride). The amount of protein was quantified by a colorimetric assay (Bio-Rad, Hercules, CA, USA). Fifty micrograms of total proteins from cell lysates were separated by SDS-PAGE and transferred onto a nitrocellulose membrane (Promega). The membrane was blotted in 5% bovine serum albumin (BSA)-Tris TBS containing 0.1% Tween 20 and probed with antibodies against E-cadherin, ERK, phosphorylated ERK (P-ERK) or β-actin (Santa Cruz). The signals were developed by using an enhanced chemiluminescence system (Pierce, Rockford, IL, USA).
Cell proliferation analysis
SKOV-3 cells transfected with CDH1 siRNA or HK, untransfected cells incubated with or without SHE 78–7 (20 μg/mL) were prepared separately for proliferation assay. The Cell Counting Kit-8 (CCK-8, Dojindo Molecular Technologies, Shanghai, China) was used to measure cell proliferation with an enzyme-labeled minireader (Bio-Rad, Japan) at 450 nm at 24, 48, 72 and 96 h. For the colony-formation assay, approximately 0.5×102 cells which had been transfected by plasmids, including the siRNA or HK, were seeded into each well of six-well plates and cultured for two weeks. Certain control cells were incubated simultaneously with SHE 78–7 (20 μg/mL) or PD98059 (50 μmol/L), respectively. Colonies were stained with gentian violet and counted under a microscope. All the experiments were done three times in triplicate wells.
Statistical analysis
All experiments were repeated at least three times. For Western blot, a representative blot from three independent experiments is shown. For numbers of cells and colonies, data were expressed as mean value±standard deviation (SD) and statistically analyzed with student's t-test, and a difference was considered significant at P<0.05. Data were analyzed using Graphpad Prism 4.
Results
E-cadherin expression was down-regulated markedly after the RNAi targeting CDH1 gene
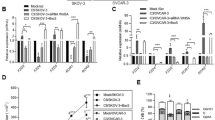
We performed RNAi targeting the CDH1 gene in SKOV-3 cells that had been reported to highly express E-cadherin22. We found that the level of E-cadherin was markedly down-regulated in SKOV-3 cells (Figure 1A). As shown in Figure 1B, E-cadherin staining was intense and restricted to cell-cell contacts in HK, the negative group, as the anti-E-cadherin antibody used for immunofluorescence recognized the intracellular tail of E-cadherin. Meanwhile, the fluorescence signal was much weaker after CDH1 gene was knocked-down via RNA interference (Figure 1B).
RNA interference of CDH1 gene inhibits E-cadherin expression in SKOV-3 cells. (A) Western blot assay shows that E-cadherin protein level in SKOV-3 cells was markedly reduced 48 h after the transfection of CDH1-siRNA plasmids compared with control plasmid HK. (B) Immunofluorescence staining assay using anti-E-cadherin antibody demonstrates the decrease of E-cadherin protein staining on the cell membrane after RNA interference of CDH1 (magnification of ×40). The scale bar stands for 20 μm.
Growth and proliferation of SKOV-3 cells was efficiently suppressed after knocking-down of E-cadherin expression
We then studied whether down-regulation of E-cadherin may affect the behavior of SKOV-3 cells, using both CCK8 and colony formation assay. As shown in Figure 2, growth of SKOV-3 cells transfected with CDH1 siRNA was suppressed at all time points (24, 48, 72, and 96 h) compared to the negative control SKOV-3-HK cells (P<0.05 for all). Also as shown in Figure 2, there was marked suppression of cell growth in the positive control cells incubated with SHE 78-7, an E-cadherin neutral antibody, at all time points compared to the SKOV-3-HK cells (P<0.05 for all).
Shutdown of E-cadherin inhibits the proliferation of SKOV-3 cells. After transfection of the CDH1-siRNA and the control plasmids, the proliferation ability of SKOV-3 was monitored by CCK8 assay, at 0, 24, 48, 72 and 96 h, respectively. SKOV-3 cells incubated in the presence of SHE 78-7, a neutral antibody of E-cadherin, were treated in the same way. Assay for every time points was repeated 3 times and the mean±SD of the OD reading value was shown in the figure. The difference of OD values between the control group and CDH1-siRNA group or SHE 78-7 group at every time point was analyzed by student's t-test. bP<0.05 compared with the HK group. eP<0.05 compared with the CDH1-siRNA group.
Marked inhibition of colony formation ability was also detected. As shown in Figure 3A, colony numbers decreased significantly in the CDH1-RNAi group, SHE 78-7 group and PD98059 (a MEK1 specific inhibitor) group in contrast to the HK group respectively (P<0.01 for all). The colony numbers in RNAi group were less than both the SHE 78-7 group (P>0.05) and PD98059 group (P<0.05), but only the latter had a statistical difference (Figure 3B). The size difference of cancer cell colony formed between the two groups (CDH1-RNAi vs HK) was also noticeable, because the colonies shrank and included much fewer cells after E-cadherin was knocked-down by RNAi (Figure 3C).
Inhibition of E-cadherin or MEK suppresses the colony formation ability of SKOV-3 cells. (A, B) Representative result and the quantitative analysis of colony formation assay in SKOV-3 cells showed the obviously decreased colony numbers in not only CDH1-siRNA group but also the SHE 78-7 group and PD98059 group, in contrast to the HK group. Mean±SD of three independent experiments was shown. PD98059, a MEK1 inhibitor. bP<0.05 compared with the HK group, eP<0.05 compared with the CDH1-siRNA group. (C) Colony of SKOV3 cells 48 h after transfection with CHD1-siRNA plasmid displayed the smaller sizes and contained much less cells compared with HK group, the negative control (magnification of ×10). The scale bar stands for 200 μm.
E-cadherin promotes ovarian cancer cell proliferation through MEK/ERK signaling pathway
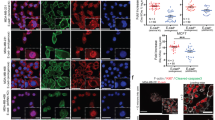
To investigate the underlying mechanism that how E-cadherin functioned to affect the proliferation and survival of ovarian cancer cells, we used a calcium-dependent E-cadherin-mediated cell-cell adhesion model21. In SKOV-3 cells, E-cadherin-mediated Ca2+-dependent cell-cell adhesion can be disrupted by a calcium chelator EGTA, and then the cell-cell adhesion can be restored by addition of physiological concentration (1.8 mmol/L) of fresh calcium solution. As shown in Figure 4A, the level of phosphorylated ERK was low in SKOV-3 cells after calcium depletion (Figure 4A, lanes 1 and 2). The restoration of calcium-dependent cell-cell adhesion, however, resulted in a rapid increase on the level of phosphorylated ERK, which lasted for nearly 20 min, and then gradually decreased to the basal level at 30 min after restoration (Figure 4A, lanes 3–6). However, when cells were pretreated with an E-cadherin-specific neutralizing antibody SHE 78-7 (20 μg/mL) for 30 min and then restored with fresh calcium for 10 min, the phosphorylation of ERK could hardly be detected (Figure 4B, lane 7). This result indicated a role of E-cadherin to activate the ERK signaling pathway. Meanwhile, no change was detected in the levels of total ERK protein regardless of any treatments in our experiment (Figure 4A and 4B). These data demonstrates that E-cadherin-mediated calcium-dependent cell-cell adhesions activate ERK signaling by the phosphorylation of ERK, but they do not modify the expression of ERK protein in ovarian cancer cells.
Cell-adhesion-mediated MEK/ERK activation is E-cadherin dependent in SKOV-3 cells. (A) E-cadherin-mediated cell adhesion activates ERK during the time course of calcium restoration in SKOV-3 cells. P-ERK, phosphorylated ERK. T-ERK, total ERK. (B) E-cadherin is required for cell-cell adhesion mediated MEK-ERK activation. Wort, wortmannin (1 μmol/L). H89, PKA inhibitor, 10 μmol/L in concentration. PD, PD98059, MEK1 inhibitor, 50 μmol/L in concentration. SHE 78-7, E-cadherin-specific antibody, 20 μg/mL in concentration. (C, D) After E-cadherin expression was inhibited by siRNA, MEK/ERK failed to be activated after calcium restoration in SKOV-3 cells, for neither ERK (C) nor MEK (D, lane 4) was observed to be phosphorylated. P-MEK, phosphorylated MEK. T-MEK, total MEK.
To examine how E-cadherin mediates the activation of ERK, cells were pretreated with several kinase inhibitors separately before restoration of calcium-containing media. As shown in Figure 4B, the phosphorylation of ERK was blocked by the pretreatment of the cells with the MEK1 specific inhibitor PD98059 (Figure 4B, lane 6), while it was not affected by the PI3K inhibitor wortmannin (Figure 4B, lane 4) or PKA inhibitor H89 (Figure 4B, lane 5). To further determine whether MEK activation is also mediated by E-cadherin, we examined the possible alteration of MEK phosphorylation after CDH1 was knocked-down. The results showed that, the same as the activation of its downstream protein ERK, MEK phosphorylation was both calcium dependent (Figure 4D, lane 3) and E-cadherin dependent, for MEK failed to be activated in the CDH1-knocked-down SKOV-3 cells even after calcium restoration (Figure 4D, lane 4). As shown in Figure 4C, no activation of ERK was observed during the disruption and restoration course of cell-cell adhesion after the expression of E-cadherin was suppressed by RNAi. Hence, we suggest that E-cadherin-mediated MEK/ERK activation may act as a major mechanism for ovarian caner cell survival and proliferation.
Discussion
To elucidate the functional implications of the distinct expression pattern of E-cadherin in ovarian cancer cells, we first established a calcium-dependent cell-cell adhesion model in E-cadherin expressing ovarian cancer cell lines such as SKOV-3 and CAOV-3 (data not shown) by a published method21. Then we knocked down E-cadherin expression by RNAi to provide a platform to investigate the role of E-cadherin in activating intracellular proliferation and survival-related signals in ovarian cancer cells. We found that down-regulation of E-cadherin expression resulted in marked suppression of survival and of colony formation abilities in ovarian cancer cells. As excessive proliferation and growth are characteristics of malignant cells, we suggest that E-cadherin may play an important role in the growth and proliferation of ovarian carcinoma. It has been previously determined in ovarian cancer that E-cadherin-mediated cellular adhesions and the successive activation of proliferation-related signaling may enhance the transformation of a single layer of normal epithelial cells to neoplastic cells, as well as the stimulation of tumor growth14. Therefore, our results suggest that E-cadherin may function as a tumor proliferation enhancer in the development of ovarian epithelial cancers.
Consistently, the up-regulation of E-cadherin expression has been considered as a new epithelial feature for OSE at the early stages of neoplastic transformation23. The E-cadherin expression is diminished at later stages of tumor progression as cells de-differentiate and acquire mesenchymal properties associated with a more aggressive phenotype23, 24, 25. Furthermore, a growing volume of data indicate that cell-cell adhesion mediated by E-cadherin is involved in the suppression of anoikis and maintenance of cellular survival in both epithelial cells, such as normal enterocytes26, oral squamous carcinoma cells12 and nonepithelia such as Ewing tumor cells27. Instead of distant hematogenous metastasis like most other tumors, regional growth and superficial invasion restricted in the peritoneal cavity are the prominent characteristics of ovarian epithelial cancer. It may result from the formation of E-cadherin-dependent multicellular spheroids, which could manifest a cell-cell adhesion mediated survival and transit to a second anchoring site even in the absence of ascites after detaching from the extracellular matrix (ECM)28.
Meanwhile, our results convincingly indicate that in SKOV-3 cells the activation of MEK/ERK triggered by cell-cell adhesion is E-cadherin-dependent, evidenced by the fact that both siRNA targeting E-cadherin and its specific neutralizing antibody are able to markedly prevent the phosphorylation of MEK or ERK. We suggest that the activation of proliferation and survival-related signal pathways of E-cadherin may be a ubiquitous phenomenon in ovarian cancer cells. Previously E-cadherin has been reported to activate MEK/ERK in squamous cell carcinoma via physically interacting with and activating EGFR29. E-cadherin may also contribute to PI3K-AKT activation by directly engaging the PI3K-p85 regulatory subunit to the adherens junctions of ovarian carcinoma cells22. Although in the current study we have no evidence to demonstrate that E-cadherin can directly interact with EGFR or MEK in ovarian cancer cells, our results strongly demonstrated that E-cadherin mediated activation of MEK/ERK exists in many E-cadherin expressing ovarian carcinoma cells such as SKOV-3 and OVCAR-317. Upon activation, MEK/ERK is known to sustain cell survival and proliferation via phosphorylating a series of downstream molecules. The activation of EGFR/MEK/ERK signaling has been demonstrated to support survival in squamous cell carcinoma cells by inducing the anti-apoptotic protein Bcl-229. In addition, MEK/ERK singling has been reported to help cells escape anoikis and maintain anchorage-independent growth in several ovarian cancer cell lines which are deprived of ECM in an in vitro environment that intimates the conditions of metastatic cells detached from ovaries and able to survive in the ascites of ovarian cancer patients30. These reports may partially explain the underlying mechanism of how E-cadherin promotes ovarian cancer cell survival and proliferation, which may help to clarify the unique role of E-cadherin in ovarian cancer progression. We suggest that E-cadherin plays a pro-oncogenic role during the development and progression of ovary cancer via a cell-cell adhesion activated MEK/ERK signaling pathway, which may serve as a potential therapeutic target to be exploited for ovarian cancer treatment in future.
Although research findings have been accumulating to reveal the ubiquitous expression of E-cadherin and its exact role in ovarian cancers, there are still many opposite opinions20 as well as lots of unsolved questions. For instance, Sawada reported that loss of E-cadherin promotes ovarian cancer cells metastasis via alpha 5-integrin31. Undoubtedly E-cadherin acts as an inhibitor in tumor metastasis, mainly for which it has been traditionally regarded as a tumor suppressor. But the relationship between its proliferation enhancer and metastasis inhibitor roles, in other words, the final counterbalance outcome of its two contrary functions, is still not well-understood. In our colony formation assay, CDH1 knocked-down SKOV-3 cells had suppressed colony formation ability, even though they 'should' tend to migrate after escaping from the E-cadherin-mediated adhesion of neighbor cells. Similarly in HT29 cells as reported, colony formation ability diminished remarkably after E-cadherin was blocked by SHE 78-732. Consistently, it is reported that Ewing tumor cells transfected by a dominant negative form of E-cadherin plasmid formed fewer and significantly smaller colonies compared with non-transduced cells27. Therefore, further research needs to be performed to try to elucidate the exact function of E-cadherin in ovarian cancer development and progression, especially the relationship between its contrary roles in regulating cell mobility and cell viability.
Abbreviations
MEK, mitogen-activated protein kinase kinase; ERK, extracellular signal-related kinase; EGFR, epidermal growth factor receptor; PKA, cAMP-dependent protein kinase A; PI3K, phosphoinositide-3 kinase; OSE, ovarian surface epithelium.
Author contribution
Lian LIU, Chun-hong MA and Li-hui HAN designed the research; Ling-ling DONG, Ji-sheng LI, Chao DU and Shan XU performed the research; Li LI and Xiu-wen WANG contributed analytic tools; Lian LIU and Ji-sheng LI analyzed data and wrote the paper.
References
Fang K, Mukhopadhyay T, Shih SH . Retinoic acid modulates epidermal growth factor receptor expression in human lung epithelial cancer cells. J Biomed Sci 1995; 2: 256–62.
Kemler R . From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet 1993; 9: 317–21.
Takeichi M . Cadherin cell adhesion receptors as a morphogenetic regulator. Science 1991; 251: 1451–5.
Aberle H, Schwartz H, Kemler R . Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem 1996; 61: 514–23.
Gottardi CJ, Wong E, Gumbiner BM . E-cadherin suppresses cellular transformation by inhibiting beta-catenin signaling in an adhesion-independent manner. J Cell Biol 2001; 153: 1049–60.
Birchmeier W, Weidner KM, Hulsken J, Behrens J . Molecular mechanisms leading to cell junction (cadherin) deficiency in invasive carcinomas. Semin Cancer Biol 1993; 4: 231–9.
Birchmeier W . E-cadherin as a tumor (invasion) suppressor gene. Bioessays 1995; 17: 97–9.
Birchmeier W, Behrens J . Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta 1994; 1198: 11–26.
Thiery JP . Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002; 2: 442–54.
Mittari E, Charalabopoulos A, Batistatou A, Charalabopoulos K . The role of E-cadherin/catenin complex in laryngeal cancer. Exp Oncol 2005; 27: 257–61.
Fearon ER . Connecting estrogen receptor function, transcriptional repression, and E-cadherin expression in breast cancer. Cancer Cell 2003; 3: 307–10.
Kantak SS, Kramer RH . E-cadherin regulates anchorage-independent growth and survival in oral squamous cell carcinoma cells. J Biol Chem 1998; 273: 16953–61.
Andrews NA, Jones AS, Helliwell TR, Kinsella AR . Expression of the E-cadherin-catenin cell adhesion complex in primary squamous cell carcinomas of the head and neck and their nodal metastases. Br J Cancer 1997; 75: 1474–80.
Sundfeldt K, Piontkewitz Y, Ivarsson K, Nilsson O, Hellberg P, Brannstrom M, et al. E-cadherin expression in human epithelial ovarian cancer and normal ovary. Int J Cancer 1997; 74: 275–80.
Maines-Bandiera SL, Auersperg N . Increased E-cadherin expression in ovarian surface epithelium: an early step in metaplasia and dysplasia. Int J Gynecol Pathol 1997; 16: 250–5.
Darai E, Scoazec JY, Walker-Combrouze F, Mlika-Cabanne N, Feldmann G, Madelenat P, et al. Expression of cadherins in benign, borderline, and malignant ovarian epithelial tumors: a clinicopathologic study of 60 cases. Hum Pathol 1997; 28: 922–8.
Reddy P, Liu L, Ren C, Lindgren P, Boman K, Shen Y, et al. Formation of E-cadherin-mediated cell-cell adhesion activates AKT and mitogen activated protein kinase via phosphatidylinositol 3 kinase and ligand-independent activation of epidermal growth factor receptor in ovarian cancer cells. Mol Endocrinol 2005; 19: 2564–78.
Auersperg N, Pan J, Grove BD, Peterson T, Fisher J, Maines-Bandiera S, et al. E-cadherin induces mesenchymal-to-epithelial transition in human ovarian surface epithelium. Proc Natl Acad Sci U S A 1999; 96: 6249–54.
Ong A, Maines-Bandiera SL, Roskelley CD, Auersperg N . An ovarian adenocarcinoma line derived from SV40/E-cadherin-transfected normal human ovarian surface epithelium. Int J Cancer 2000; 85: 430–7.
Lau MT, Klausen C, Leung PC . E-cadherin inhibits tumor cell growth by suppressing PI3K/Akt signaling via beta-catenin-Egr1-mediated PTEN expression. Oncogene 2011; 30: 2753–66.
Nakagawa M, Fukata M, Yamaga M, Itoh N, Kaibuchi K . Recruitment and activation of Rac1 by the formation of E-cadherin-mediated cell-cell adhesion sites. J Cell Sci 2001; 114: 1829–38.
De Santis G, Miotti S, Mazzi M, Canevari S, Tomassetti A . E-cadherin directly contributes to PI3K/AKT activation by engaging the PI3K-p85 regulatory subunit to adherens junctions of ovarian carcinoma cells. Oncogene 2009; 28: 1206–17.
Inoue M, Ogawa H, Miyata M, Shiozaki H, Tanizawa O . Expression of E-cadherin in normal, benign, and malignant tissues of female genital organs. Am J Clin Pathol 1992; 98: 76–80.
Veatch AL, Carson LF, Ramakrishnan S . Differential expression of the cell-cell adhesion molecule E-cadherin in ascites and solid human ovarian tumor cells. Int J Cancer 1994; 58: 393–9.
Davies BR, Worsley SD, Ponder BA . Expression of E-cadherin, alpha-catenin and beta-catenin in normal ovarian surface epithelium and epithelial ovarian cancers. Histopathology 1998; 32: 69–80.
Fouquet S, Lugo-Martinez VH, Faussat AM, Renaud F, Cardot P, Chambaz J, et al. Early loss of E-cadherin from cell-cell contacts is involved in the onset of Anoikis in enterocytes. J Biol Chem 2004; 279: 43061–9.
Kang HG, Jenabi JM, Zhang J, Keshelava N, Shimada H, May WA, et al. E-cadherin cell-cell adhesion in ewing tumor cells mediates suppression of anoikis through activation of the ErbB4 tyrosine kinase. Cancer Res 2007; 67: 3094–105.
Lengyel E . Ovarian cancer development and metastasis. Am J Pathol 2010; 177: 1053–64.
Shen X, Kramer RH . Adhesion-mediated squamous cell carcinoma survival through ligand-independent activation of epidermal growth factor receptor. Am J Pathol 2004; 165: 1315–29.
Al-Ayoubi A, Tarcsafalvi A, Zheng H, Sakati W, Eblen ST . ERK activation and nuclear signaling induced by the loss of cell/matrix adhesion stimulates anchorage-independent growth of ovarian cancer cells. J Cell Biochem 2008; 105: 875–84.
Sawada K, Mitra AK, Radjabi AR, Bhaskar V, Kistner EO, Tretiakova M, et al. Loss of E-cadherin promotes ovarian cancer metastasis via alpha 5-integrin, which is a therapeutic target. Cancer Res 2008; 68: 2329–39.
Green SK, Francia G, Isidoro C, Kerbel RS . Antiadhesive antibodies targeting E-cadherin sensitize multicellular tumor spheroids to chemotherapy in vitro. Mol Cancer Ther 2004; 3: 149–59.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant No 30772329 and 81172487), China Postdoctoral Science Foundation (grant No 20090450153), Grant for Postdoctoral Researchers with Creative Projects of Shandong Province, China (No 200801009) and Natural Science Foundation of Shandong Province, China (grant No ZR2009CM004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, Ll., Liu, L., Ma, Ch. et al. E-cadherin promotes proliferation of human ovarian cancer cells in vitro via activating MEK/ERK pathway. Acta Pharmacol Sin 33, 817–822 (2012). https://doi.org/10.1038/aps.2012.30
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2012.30
Keywords
This article is cited by
-
Paleotectonic stress field modeling and fracture prediction of the Longmaxi Formation in the N216 well block, Southern Sichuan Basin, China
Arabian Journal of Geosciences (2022)
-
Effects of mechanical layering on hydraulic fracturing in shale gas reservoirs based on numerical models
Arabian Journal of Geosciences (2018)
-
E-cadherin expression is correlated with focal adhesion kinase inhibitor resistance in Merlin-negative malignant mesothelioma cells
Oncogene (2017)
-
Construction and characteristics of an E-cadherin-related three-dimensional suspension growth model of ovarian cancer
Scientific Reports (2014)
-
RETRACTED ARTICLE: Aberrant promoter methylation of the CHD1 gene may contribute to the pathogenesis of breast cancer: a meta-analysis
Tumor Biology (2014)