Abstract
Aim:
Pirarubicin (THP) is recently found to be effective in treating patients with advanced, relapsed or recurrent high-grade osteosarcoma. In this study, the effects of THP on the multidrug-resistant (MDR) osteosarcoma cells were assessed, and the underlying mechanisms for the disruption of cell cycle kinetics by THP were explored.
Methods:
Human osteosarcoma cell line MG63 and human MDR osteosarcoma cell line MG63/DOX were tested. The cytotoxicity of drugs was examined using a cell proliferation assay with the Cell Counting Kit-8 (CCK-8). The distribution of cells across the cell cycle was determined with flow cytometry. The expression of cell cycle-regulated genes cyclin B1 and Cdc2 (CDK1), and the phosphorylated Cdc2 and Cdc25C was examined using Western blot analyses.
Results:
MG63/DOX cells were highly resistant to doxorubicin (ADM) and gemcitabine (GEM), but were sensitive or lowly resistant to THP, methotrexate (MTX) and cisplatin (DDP). Treatment of MG63/DOX cells with THP (200–1000 ng/mL) inhibited the cell proliferation in time- and concentration-dependent manners. THP (50–500 ng/mL) induced MG63/DOX cell cycle arrest at the G2/M phase in time- and concentration-dependent manners. Furthermore, the treatment of MG63/DOX cells with THP (200–1000 ng/mL) downregulated cyclin B1 expression, and decreased the phosphorylated Cdc2 at Thr161. Conversely, the treatment increased the phosphorylated Cdc2 at Thr14/Tyr15 and Cdc25C at Ser216, which led to a decrease in Cdc2-cyclin B1 activity.
Conclusion:
The cytotoxicity of THP to MG63/DOX cells may be in part due to its ability to arrest cell cycle progression at the G2/M phase, which supports the use of THP for managing patients with MDR osteosarcoma.
Similar content being viewed by others
Introduction
Osteosarcoma is the most common malignant primary bone tumor in children, adolescents and young adults. Multiagent chemotherapy, commonly including doxorubicin (ADM), cisplatin (DDP) and high-dose methotrexate (MTX), has improved patient survival from 11% with surgical resection alone to 70% for localized disease. Unfortunately, the long-term survival for the remaining patients with recurrent disease is less than 20%1, 2, 3. Studies designed to identify novel active agents and implement strategies to overcome chemoresistance will likely be important for improving survival1.
Recently, pirarubicin (THP), a novel anthracycline derivative of ADM, has been used clinically to treat tumors such as osteosarcoma, breast cancer, lymphoma and acute myeloid leukemia4, 5, 6, 7. Moreover, THP has shown a greater antitumor activity8, 9, 10 and lower cardiotoxicity11 than ADM. This may be explained by the higher uptake of THP by tumor cells than ADM and its rapid distribution into the nucleus and subsequent incorporation into deoxyribonucleic acid (DNA)12, 13, 14. More recently, we found that through retrospective analysis, THP-based chemotherapy regimens were effective and safe as a salvage chemotherapy option for patients with lung metastases, refractory or recurrent high-grade osteosarcoma who previously received adjuvant chemotherapy with high dose-MTX–DDP–ADM–ifosfamide15, 16. However, the exact mechanisms by which THP exerts its antitumor effects are not understood. Although previous studies on THP have revealed that induction of cell cycle arrest at the G2 phase may contribute to its action in RPMI-8402 cells17, the molecular basis of the cell cycle arrest induced by THP remains unclear.
Considering this previous research, we speculated that THP might be a potential chemotherapeutic agent that can circumvent drug resistance in patients with osteosarcoma. However, few studies clearly define the effects of THP on cytotoxicity and multidrug-resistant (MDR) osteosarcoma cells. In this study, we investigated the in vitro cytotoxic response of the MDR osteosarcoma cell line MG63/DOX treated with THP and explored the underlying mechanisms THP utilizes to disrupt cell cycle kinetics.
Materials and methods
Reagents
THP was obtained from Wan Le Pharma (Shenzhen, China); ADM and MTX, from Pfizer Pharma (New York, NY, USA); gemcitabine (GEM), from Lilly Pharma (Saint-Cloud, France); and DDP, from Hao Shen Pharma (Nanjing, China). Propidium iodide (PI) was purchased from Sigma Chemicals (St Louis, MO, USA). Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Laboratories (Kumamoto, Japan).
Cell lines and cell culture
The human osteosarcoma parental cell line, MG63, was obtained from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). The human MDR osteosarcoma cell line MG63/DOX, which overexpresses P-glycoprotein (P-gp) and was selected in a step-wise manner by exposing drug-sensitive MG63 cells to increasing doses of ADM, was kindly provided by Dr Yoshio ODA (Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan)18. The cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Hyclone, Logan, UT, USA) supplemented with 10% heat-inactivated fetal calf serum (FCS; Si Ji Qing, Hangzhou, China), 100 units/mL penicillin and 100 mg/mL streptomycin (Gibco, Grand Island, NY, USA) in a humidified atmosphere at 37 °C consisting of 5% CO2. Drugs were initially dissolved in phosphate-buffered saline (PBS) and then serially diluted in culture medium to the desired drug treatment concentrations.
Drug sensitivity and cytotoxicity assays
The effects of THP, ADM, MTX, DDP, and GEM on the proliferation of MG63/DOX and MG63 cells were measured using the CCK-8 colorimetric assay. Briefly, the cells were seeded in a 96-well microtiter plate at 5×103 cells/well (100 μL). After 24 h of incubation with fresh medium, 10 μL of the various chemical dilutions at the indicated concentrations of each drug was added to the plates, and the cells were incubated for an additional 24, 48, and 72 h. At the end of drug treatment, 10 μL of CCK-8 was added to each well, and the cells were incubated for 4 h at 37 °C. Absorbance (A) was analyzed on a 96-well Opsys MR Microplate Reader (Thermo Labsystems, Beverly, MA, USA) at 450 nm. All experiments were tested in triplicate and repeated at least three times. The resistance factor (R factor) of multidrug-resistant cell line MG63/DOX for a particular drug is defined as the ratio of IC50 of MG63/DOX cell to IC50 of MG63 cell at 72 h (R<5×: low or no-resistance; R 5–15×: moderate-resistance; R>20×: high-resistance)19.
Cell cycle analysis
MG63/DOX cells were treated with THP for 24, 48, and 72 h at concentrations of 50, 200, and 500 ng/mL. Control cells were treated with solvent alone for the durations indicated above. Cell cycle was analyzed as previously described20. The cells were trypsinized, washed twice with ice cold PBS, fixed in 70% ethanol and stained with propidium iodide (PI; 5 μg/mL PI in PBS containing 0.1% Triton X-100 and 0.2 mg/mL RNase A) in the dark for 30 min at 4 °C. Finally, the cells were analyzed for cell cycle perturbation using a FACSCalibur flow cytometer (Becton-Dickinson, San Diego, CA, USA). Cell fluorescence was measured in duplicate at each time point, and all experiments were performed in triplicate.
Western blot analysis
Cells treated with THP at the indicated concentrations were harvested following 72 h of incubation. Western blotting was performed as described previously21. Briefly, 30 μg of protein from whole-cell lysates was separated on a sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) apparatus and electrotransferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA). After blocking with 5% (w/v), non-fat dry milk in Tris-buffered saline for 1 h at room temperature, membranes were incubated overnight at 4 °C with the previously described pretreated antibody diluent according to the one-step Western kit manufacturer's instructions. Primary antibodies were incubated with a horseradish peroxidase antibody for 5 min at room temperature and then diluted (1:1000). The protein bands were visualized using a chemiluminescence imaging system (Bio-Rad, Hercules, CA, USA). All blots are representative of three independent experiments. Primary antibodies assayed were Cyclin B1, Cdc2, p-Cdc2 (Thr14), p-Cdc2 (Tyr15), and p-Cdc2 (Thr161) antibodies (Cell Signaling Technology, Boston, MA, USA); Cdc25C and p-Cdc25C (Ser216) antibodies (Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA); and β-actin antibody (CoWin Biotech Co, Beijing, China).
Statistical analysis
Data are presented as the mean±SD. The Student's t-test was used to analyze the difference between the mean values of the treatment and the control groups. Differences with a P value of less than 0.05 were considered statistically significant.
Results
Drug sensitivity
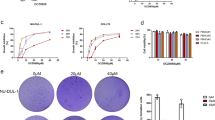
We investigated the effects of THP and chemotherapeutic agents commonly used for osteosarcoma treatment, including ADM, MTX, DDP, and GEM, on the proliferation of MG63/DOX MDR human osteosarcoma cells and their parental MG63 cells using the CCK-8 colorimetric assay. As shown in Table 1, MG63/DOX cells exhibited high levels of resistance to ADM (R factor: 121.6) and GEM (R factor: 108.3) but little to no resistance to THP (R factor: 3.73), MTX (R factor: 4.16) and DDP (R factor: 0.91). These results indicate that MG63/DOX cells reveal have a classic MDR phenotype, which has been previously described18. Surprisingly, THP had similar inhibitory effects on cell proliferation in both resistant and parental osteosarcoma cells, indicating that MDR osteosarcoma cells are still sensitive to THP.
THP inhibited proliferation of MG63/DOX cells
The cytotoxic effects of THP on MG63/DOX cells were further measured with the CCK-8 colorimetric assay after the cells were exposed to various concentrations of THP for multiple durations. Cell growth was inhibited in a concentration- and time-dependent manner (Figure 1 and Table 2).
Effect of THP on cell proliferation of MG63/DOX cells. MG63/DOX cells were treated with THP at the indicated concentrations (200, 500, and 1000 ng/mL) for 24–72 h. Cell proliferation was assessed by CCK-8 assay, and cell proliferation values were expressed relative to those of the untreated cells (100% control value). THP inhibited the proliferation of MG63/DOX cells in a time and concentration-dependent manner (reference Table 2). Mean±SD. n=3.
THP induced MG63/DOX cells cycle arrest at the G2/M phase
To examine the mechanism of inhibition of cell growth by THP, cell cycle perturbation was evaluated by flow cytometry after the MG63/DOX cells were exposed to THP at various concentrations for 24, 48, or 72 h. As shown in Figure 2 and Table 3, the fraction of cells in the G2/M phase increased with the concentration of THP and the duration of treatment. A concurrent reduction in the proportion of cells in G0/G1 and S phase was observed. These results demonstrate that THP induced a cycle arrest in MG63/DOX cells at G2/M phase in a time- and concentration-dependent manner.
Effect of THP on cell cycle distribution of MG63/DOX cells. MG63/DOX cells were treated with the indicated concentrations (50, 200, and 500 ng/mL) of THP and harvested at 24, 48, or 72 h. DNA content was analyzed by flow cytometry using PI staining. THP induced MG63/DOX cells cycle arrest at the G2/M phase time and dose-dependently (reference Table 3). A representative profile is shown for each treatment.
THP reduced cyclin B1 expression and Cdc2 and Cdc25C activity
To elucidate the molecular basis for THP-induced cell cycle arrest at the G2/M phase, Cyclin B1, and Cdc2 protein expression was assayed by Western blotting. As shown in Figure 3, the results indicate that protein levels of cyclin B1 decreased after THP treatment in a time- and concentration-dependent manner but that the total protein levels of Cdc2 did not change. The Cdc2-cyclin B1 complex was retained in an inactive state by the negative phosphorylation of the residues Thr14 and Tyr15 on Cdc2 phosphorylated by kinase Wee1 and Myt1, and Cdc2-cyclin B1 activity is increased by the phosphorylation of Cdc2 at Thr161. Therefore, we examined Cdc2 phosphorylation by Western blotting and found that while the protein expression level of Cdc2 was not altered, the phosphorylation of Cdc2 at Thr14/Tyr15 [p-Cdc2 (Thr14/Tyr15)] was increased and the phosphorylation of Cdc2 at Thr-161 [p-Cdc2 (Thr161)] was decreased after THP treatment in a time- and concentration-dependent manner. These data suggest that reduced Cdc2-cyclinB1 activity by THP may account for the G2/M arrest in our model.
Effects of THP on the protein levels and activity of cell cycle regulatory proteins in MG63/DOX cells. MG63/DOX cells were treated THP at the indicated concentrations (200, 500, and 1000 ng/mL). Cellular proteins were extracted at the indicated time (24, 48, or 72 h) following drug exposure. A total of 30 μg cell extract protein isolated from the drug-treated and untreated MG63/DOX cells was subjected to Western-blotting with antibodies against cyclin B1, Cdc2, p-Cdc2 (Thr14/Tyr15), p-Cdc2 (Thr161), Cdc25C, and p-Cdc25C (Ser216). β-actin was used as a loading control. Densitometry assay showed that the protein levels of cyclin B1 was decreased after THP treatment in time- and concentration-dependantly and the total protein level of Cdc2 was not changed, but the phosphorylation of Cdc2 at Thr14/Tyr15 [p-Cdc2 (Thr14/Tyr15)] were increased and the phosphorylation of Cdc2 at Thr161 [p-Cdc2 (Thr161)] was decreased after THP treatment in a time- and concentration-dependant manner and the total protein level of Cdc25C were not changed, but the phosphorylation of Cdc25C at Ser216 [p-Cdc25C (Ser216)] was increased after THP treatment in a time and concentration-dependant manner (data not shown). Western blot data presented are representative of those obtained from three separate experiments.
Cdc25C activates Cdc2 by removing inhibitory phosphate groups on Thr14 and Tyr15. Because Cdc2 phosphorylation at Thr14/Tyr15 was enhanced by THP, we further investigated the effect of THP on Cdc25C expression and phosphorylation at Ser216 by Western blotting. We found that the total protein expression levels of Cdc25C were not altered but that the phosphorylation of Cdc25C at Ser216 [p-Cdc25C (Ser216)] was increased after THP treatment in a time- and concentration-dependent manner (Figure 3). These results suggest that decreased dephosphorylation by Cdc25C is partly responsible for Cdc2 inactivation.
Discussion
Currently, one of the greatest obstacles to improving the survival of patients with osteosarcoma is acquired clinical resistance to chemotherapeutic agents, primarily to the three most widely used agents in the treatment of osteosarcoma — ADM, MTX, and DDP1, 22, 23. Cancer cells can utilize a number of different mechanisms to become resistant to one or more chemotherapeutic drugs. Depending on the drug and cellular context, factors such as drug inactivation, drug target mutation, drug target upregulation and downregulation, decreased drug uptake, increased drug elimination and DNA damage repair have all been shown to contribute to both intrinsic and acquired resistant to chemotherapy1. ADM is one of the most effective agents for osteosarcoma treatment1, 22, 23. Although resistance to ADM in osteosarcoma is likely to be multifactorial, P-gp is thought to be the main resistance mechanism against this agent1, 24. Additionally, some retrospective studies25, 26 have revealed that the overexpression of P-gp may be associated with poor prognosis. THP, a semisynthetic anthracycline glycoside, is a newer generation anthracycline anticancer agent that is reported to have a lower cardiotoxicity than ADM11. Changes in structure allow THP to be taken up by tumor cells approximately 170 times faster than ADM and increase the rates at which it distributes into the nucleus and intercalates into DNA12, 13, 14.
On the basis of these considerations, we tested THP for cytotoxicity against MDR osteosarcoma MG63/DOX cells in vitro. We found that THP has a marked inhibitory effect on cell proliferation in both resistant and sensitive osteosarcoma cells. These results suggest that THP partially overcomes the resistance caused by overexpression of P-gp and that THP may play an important role in treatment of patients with refractory or recurrent high-grade osteosarcoma. In fact, THP has also shown favorable activity in various types of cancer cells, including P-gp overexpressing breast cancer27, ADM-resistant lymphoblastoma28, MG-63 cells29 and bladder cancers30 in vitro, and has substantial clinical activity against various tumors without severe side effects4, 5, 6, 7, 15, 16. Despite the ability of THP to overcome MDR in clinical studies6, 16, 17, very little is known about THP-induced cytotoxicity and the underlying mechanisms of pirarubicin on MDR osteosarcoma cells. To our knowledge, this is the first study to evaluate the cytotoxicity of THP against human drug-resistant osteosarcoma cells.
In addition, we demonstrated that the effect of THP in resistant MG63/DOX cells is associated with cell cycle arrest at the G2/M phase in a time- and concentration-dependent manner. Similarly, Takamoto et al17 reported that THP exerted its growth-inhibitory effect by blocking RPMI-8402 cells irreversibly at G2 and that the G2 phase accumulation was a cytocidal effect of THP. The eukaryotic cell cycle is strictly regulated by a class of cyclins and cyclindependent kinases (CDKs)31. The progression from G2 to mitosis is controlled by the mitosis-promoting factor, which comprises a complex of Cdc2 (CDK1) and cyclin B32, 33, 34, 35. During G2/M transition, CDK1 activation requires an association with cyclin B1 and phosphorylation at Thr161 by CDK-activating kinase (CAK), whereas the CDK1/cyclin B complex is kept inactive by phosphorylation on Thr14 or Tyr15 of CDK1 by kinase Wee1 and Myt136. We found that THP significantly downregulated cyclin B1, p-Cdc2 (Thr161) and upregulated p-Cdc2 (Thr14/Tyr15). Decreased cyclin B1 levels and Cdc2 phosphorylation at Thr161 and increased Cdc2 phosphorylation at Thr14/Tyr15 may contribute to the THP-induced arrest of MG63/DOX cells at the G2/M phase and the subsequent blocking of cell cycle progression. Finally, we demonstrated that THP enhanced Cdc25C phosphorylation at the Ser-216 residue in our cell cultured model. The activity of Cdc25C, which is essential for progression into mitosis, is regulated by changes in protein levels, subcellular localization and the phosphorylation state37. It is proposed that the checkpoint kinase, Chk1, regulates the interactions between human Cdc25C and 14-3-3 proteins by phosphorylating Cdc25C on serine 216. 14-3-3 proteins, in turn, function to keep Cdc25C out of the nucleus38. Therefore, the effect of THP on G2/M phase arrest in MG63/DOX cells may be partly mediated by Chk1 activation. However, future studies are required to determine whether and how THP affects Chk1 in our model system.
In conclusion, we have demonstrated an encouraging efficacy of THP against human MDR osteosarcoma cells in vitro. We also have found, for the first time, that THP could arrest the cell cycle at the G2/M phase, which was partially associated with the downregulation of cyclin B1, p-Cdc2 (Thr161), and the upregulation of p-Cdc2 (Thr14), p-Cdc2 (Tyr15), and p-Cdc25C (Ser216). The alterations of cell cycle kinetics might contribute to a better understanding of the cytotoxicity induced by THP. These findings also provide a theoretical basis for its potential use in the management of patients with MDR osteosarcoma and suggest that further in vivo and prospective clinical studies are warranted.
Author contribution
Dr Yang YAO designed the research; Dr Shui-er ZHENG designed the research, wrote the paper, analyzed the data and performed the research; Dr Sang XIONG performed the research, analyzed the data, wrote the paper and designed the research; Dr Feng LIN designed the research; Dr Guang-lei QIAO performed the research and analyzed the data; Dr Tao FENG performed the research; Dr Zan SHEN designed the research; Dr Da-liu MIN performed the research; and Dr Chun-ling ZHANG contributed new reagents.
References
Chou AJ, Gorlick R . Chemotherapy resistance in osteosarcoma current challenges and future directions. Expert Rev Anticancer Ther 2006; 6: 1075–85.
Hawkins DS, Arndt CA . Pattern of disease recurrence and prognostic factors in patients with osteosarcoma treated with contemporary chemotherapy. Cancer 2003; 98: 2447–56.
Chou AJ, Merola PR, Wexler LH, Gorlick RG, Vyas YM, Healey JH, et al. Treatment of osteosarcoma at first recurrence after contemporary therapy: the memorial sloan-kettering cancer center experience. Cancer 2005; 104: 2214–21.
Shinozaki T, Watanabe H, Yanagawa T, Shirakura K, Takagishi K . Pirarubicin-based versus doxorubicin-based osteosarcoma chemotherapy. Ann Pharmacother 2002; 36: 996–9.
Li JJ, Di GH, Tang LC, Yu KD, Hu Z, Liu GY, et al. Adjuvant therapy of breast cancer with pirarubicin versus epirubicin in combination with cyclophosphamide and 5-fluorouracil. Breast J 2011; 17: 657–60.
Kasahara S, Hara T, Tsurumi H, Goto N, Kitagawa J, Kanemura N, et al. Phase II study of the tetrahydropyranyl adriamycin-cyclophosphamide, vincristine, and prednisolone regimen combined with rituximab as first-line treatment for elderly patients with diffuse large B-cell lymphoma. Leuk Lymphoma 2011; 52: 629–34.
Kudo K, Kojima S, Tabuchi K, Yabe H, Tawa A, Imaizumi M, et al. Prospective study of a pirarubicin, intermediate-dose cytarabine, and etoposide regimen in children with down syndrome and acute myeloid leukemia: the Japanese Childhood AML Cooperative Study Group. J Clin Oncol 2007; 25: 5442–7.
Nagai K, Nagasawa K, Sadzuka Y, Tsujimoto M, Takara K, Ohnishi N, et al. Relationships between the in vitro cytotoxicity and transport characteristics of pirarubicin and doxorubicin in M5076 ovarian sarcoma cells, and comparison with those in Ehrlich ascites carcinoma cells. Cancer Chemother Pharmacol 2002; 49: 244–50.
Umezawa H, Takahashi Y, Kinoshita M, Naganawa H, Masuda T, Ishizuka M, et al. Tetrahydropyranyl derivatives of daunomycin and adriamycin. J Antibiot (Tokyo) 1979; 32: 1082–4.
Tsuruo T, Iida H, Tsukagoshi S, Sakurai Y. 4'-O-tetrahydropyranyladriamycin as a potential new antitumor agent. Cancer Res 1982; 42: 1462–7.
Dantchev D, Paintrand M, Hayat M, Bourut C, Mathé G . Low heart and skin toxicity of a tetrahydropyranyl derivate of Adriamycin (THP-ADM) as observed by electron and light microscopy. J Antibiot (Tokyo) 1979; 32: 1085–6.
Zou HY, Wu HL, Zhang Y, Li SF, Nie JF, Fu HY, et al. Studying the interaction of pirarubicin with DNA and determining pirarubicin in human urine samples: combining excitation — emission fluorescence matrices with second-order calibration methods. J Fluoresc 2009; 19: 955–66.
Sridhar KS, Hussein AM, Benedetto P, Ardalan B, Savaraj N, Richman SP . Phase II trial of 4′-0-tetrahydropyranyladriamycin (pirarubicin) in head and neck carcinoma. Cancer 1992; 70: 1591–7.
Tsurumi H, Hara T, Goto N, Kanemura N, Kasahara S, Sawada M, et al. A phase II study of a THP-COP regimen for the treatment of elderly patients aged 70 years or older with diffuse large B-cell lymphoma. Hematol Oncol 2007; 2: 107–14.
Zhao H, Yao Y, Wang Z, Lin F, Sun Y, Yao Y, et al. Therapeutic effect of pirarubicin-based chemotherapy for osteosarcoma patients with lung metastasis. J Chemother 2010; 22: 119–24.
Qi WX, He AN, Tang LN, Shen Z, Yao Y . Evaluation of pirarubicin-cisplatin chemotherapy in the treatment for refractory and recurrent high-grade osteosarcoma: experience of a single institute. Med Oncol 2011. doi: 10.1007/s12032-011-0021-y.
Takamoto S, Ota K . Flow cytometric analysis of the effect of THP-adriamycin on the cell cycle traverse of RPMI-8402 cells — comparison with adriamycin. Gan To Kagaku Ryoho 1986; 13: 1868–75.
Oda Y, Matsumoto Y, Harimaya K, Iwamoto Y, Tsuneyoshi M . Establishment of new multidrug-resistant human osteosarcoma cell lines. Oncol Rep 2000; 7: 859–66.
Snow K, Judd W . Characterisation of adriamycin- and amsacrine-resistant human leukaemic T cell lines. Br J Cancer 1991; 63: 17–28.
Guo JM, Xiao BX, Liu Q, Zhang S, Liu DH, Gong ZH . Anticancer effect of aloe-emodin on cervical cancer cells involves G2/M arrest and induction of differentiation. Acta Pharmacol Sin 2007; 28: 1991–5.
Zheng SE, Yao Y, Dong Y, Lin F, Zhao H, Shen Z, et al. Down-regulation of ribosomal protein L7A in human osteosarcoma. J Cancer Res Clin Oncol 2009; 135: 1025–31.
Bacci G, Briccoli A, Ferrari S, Longhi A, Mercuri M, Capanna R, et al. Neoadjuvant chemotherapy for osteosarcoma of the extremity: long-term results of the Rizzoli's 4th protocol. Eur J Cancer 2001; 37: 2030–9.
Chi SN, Conklin LS, Qin J, Meyers PA, Huvos AG, Healey JH, et al. The patterns of relapse in osteosarcoma: the Memorial Sloan-Kettering experience. Pediatr Blood Cancer 2004; 42: 46–51.
Cagliero E, Ferracini R, Morello E, Scotlandi K, Manara MC, Buracco P, et al. Reversal of multidrug-resistance using Valspodar (PSC 833) and doxorubicin in osteosarcoma. Oncol Rep 2004; 12: 1023–31.
Serra M, Scotlandi K, Reverter-Branchat G, Ferrari S, Manara MC, Benini S, et al. Value of P-glycoprotein and clinicopathologic factors as the basis for new treatment strategies in high-grade osteosarcoma of the extremities. J Clin Oncol 2003; 21: 536–42.
Hornicek FJ, Gebhardt MC, Wolfe MW, Kharrazi FD, Takeshita H, Parekh SG, et al. P-glycoprotein levels predict poor outcome in patients with osteosarcoma. Clin Orthop 2000; 373: 11–7.
Kubota T, Furukawa T, Tanino H, Oura S, Murata H, Yuasa S, et al. Pirarubicin might partly circumvent the P-glycoprotein-mediated drug resistance of human breast cancer tissues. Anticancer Res 1998; 18: 967–72.
Kunimoto S, Miura K, Umezawa K . Cellular uptake and efflux and cytostatic activity of 4′-O-tetrahydropyranyladriamycin in adriamycin-sensitive and resistant tumor cell lines. J Antibiot (Tokyo) 1984; 37: 1697–702.
Liu SY, Song SX, Lin L, Liu X . Molecular mechanism of cell apoptosis by paclitaxel and pirarubicin in a human osteosarcoma cell line. Chemotherapy 2010; 56: 101–7.
Maruyama T, Higuchi Y, Suzuki T, Qiu J, Yamamoto S, Shima H . Double short-time exposure to pirarubicin produces higher cytotoxicity against T24 bladder cancer cells. J Infect Chemother 2011; 17: 11–6.
Suryadinata R, Sadowski M, Sarcevic B . Control of cell cycle progression by phosphorylation of cyclin-dependent kinase (CDK) substrates. Biosci Rep 2010; 30: 243–55.
Yen CY, Chiu CC, Chang FR, Chen JY, Hwang CC, Hseu YC, et al. 4beta-Hydroxywithanolide E from Physalis peruviana (golden berry) inhibits growth of human lung cancer cells through DNA damage, apoptosis and G2/M arrest. BMC Cancer 2010; 10: 46.
Stan SD, Zeng Y, Singh SV . Ayurvedic medicine constituent withaferin a causes G2 and M phase cell cycle arrest in human breast cancer cells. Nutr Cancer 2008; 60: 51–60.
Dvory-Sobol H, Cohen-Noyman E, Kazanov D, Figer A, Birkenfeld S, Madar-Shapiro L, et al. Celecoxib leads to G2/M arrest by induction of p21 and down-regulation of cyclin B1 expression in a p53-independent manner. Eur J Cancer 2006; 42: 422–6.
Doree M, Hunt T . From Cdc2 to Cdk1: when did the cell cycle kinase join its cyclin partner? J Cell Sci 2002; 115: 2461–4.
Taylor WR, Stark GR . Regulation of the G2/M transition by p53. Oncogene 2001; 20: 1803–15.
Lopez-Girona A, Furnari B, Mondesert O, Russell P . Nuclear localization of Cdc25 is regulated by DNA damage and a 14-3-3 protein. Nature 1999; 397: 172–5.
Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica-Worms H, et al. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science 1997; 277: 1497–501.
Acknowledgements
We thank Dr Yoshio ODA for kindly providing the MG63/DOX cell line. This work was supported by grants from National Natural Science Foundation of China (Grant No 81001192 and 81172105) and Shanghai Health Bureau (Grant No 2011171).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, Se., Xiong, S., Lin, F. et al. Pirarubicin inhibits multidrug-resistant osteosarcoma cell proliferation through induction of G2/M phase cell cycle arrest. Acta Pharmacol Sin 33, 832–838 (2012). https://doi.org/10.1038/aps.2012.20
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2012.20
Keywords
This article is cited by
-
The roles and therapeutic potential of cyclin-dependent kinases (CDKs) in sarcoma
Cancer and Metastasis Reviews (2016)
-
Identification of genes associated with methotrexate resistance in methotrexate-resistant osteosarcoma cell lines
Journal of Orthopaedic Surgery and Research (2015)
-
Pirarubicin versus doxorubicin in neoadjuvant/adjuvant chemotherapy for stage IIB limb high-grade osteosarcoma: Does the analog matter?
Medical Oncology (2015)
-
Telekin suppresses human hepatocellular carcinoma cells in vitro by inducing G2/M phase arrest via the p38 MAPK signaling pathway
Acta Pharmacologica Sinica (2014)