Abstract
Aim:
Hec1, a member of the Ndc80 kinetochore complex, is highly expressed in cancers. The aim of this study was to explore the role and mechanism of action of Hec1 with respect to the cytotoxicity of paclitaxel in ovarian cancer.
Methods:
Thirty ovarian cancer samples and 6 normal ovarian samples were collected. Hec1 expression in these samples was determined with immunohistochemistry. Ovarian cancer cell lines A2780, OV2008, C13K, SKOV3, and CAOV3 and A2780/Taxol were examined. Cell apoptosis and cell cycle analysis were detected with flow cytometric technique. siRNA was used to delete Hec1 in the cells. The expression of related mRNAs and proteins was measured using RT-PCR and Western blot analysis, respectively.
Results:
Hec1 expression was significantly higher in ovarian cancer samples than in normal ovarian samples, and was associated with paclitaxel-resistance and poor prognosis. Among the 6 ovarian cancer cell lines examined, Hec1 expression was highest in paclitaxel-resistant A2780/Taxol cells, and lowest in A2780 cells. Depleting Hec1 in A2780/Taxol cells with siRNA decreased the IC50 value of paclitaxel by more than 10-fold (from 590±26.7 to 45.6±19.4 nmol/L). Depleting Hec1 in A2780 cells had no significant effect on the paclitaxel sensitivity. In paclitaxel-treated A2780/Taxol cells, depleting Hec1 significantly increased the cleaved PARP and Bax protein levels, and decreased the Bcl-xL protein level.
Conclusion:
Hec1 overexpression is associated with the progression and poor prognosis of ovarian cancer. Inhibition of Hec1 expression can sensitize ovarian cancer cells to paclitaxel.
Similar content being viewed by others
Introduction
Paclitaxel (Taxol), one of the broadest-spectrum anticancer agents, is currently used to treat patients with ovarian and breast carcinomas. The anti-tumor function of paclitaxel is to target the microtubules of the mitotic spindle to impede chromosome alignment and segregation, thereby blocking cell cycle progression and activating apoptosis pathways1. However, paclitaxel resistance is a fundamental problem in cancer management and is the primary reason for treatment failure. Recently, many resistance mechanisms have been discovered that involve proteins such as PTEN, AKT, PI3K, MDR-associated protein and various mitotic checkpoint proteins. Many researchers have reported that the elevated (in the case of Aurora Kinase A) or the decreased (for example, BubR1 and Mad2) expression of mitotic checkpoint proteins can antagonize the effects of paclitaxel2,3,4. Therefore, many molecules that interfere with the spindle assembly checkpoint could contribute to the effects of paclitaxel.
Hec1, coded by the gene Hec1, is a member of the Ndc80 kinetochore complex, which is overexpressed in cancer. As a kinetochore outer layer component and a positive spindle assembly checkpoint control, Hec1 plays an important role in the formation of stable kinetochore-microtubule interactions and in proper chromosome alignment during mitosis5,6,7. The depletion of Hec1 impairs chromosome congression and leads to the persistent activation of the spindle checkpoint6. Hec1 overexpression has been observed in numerous human cancers and was determined to be associated with worse clinical outcomes in primary breast cancer and other cancers8,9. One study suggested that inhibiting Hec1 may be an effective approach for therapeutic intervention in cancer10. In this study, we used siRNA to downregulate the expression of Hec1 in ovarian cancer cells and explored the role and mechanism of action of Hec1 pertaining to improving the cytotoxicity of paclitaxel.
Materials and methods
Cell lines and culture
The A2780 human ovarian cancer cell line was obtained from the European Collection of Cell Cultures (ECACC, Salisbury, UK). The OV2008 and C13K ovarian cancer cell lines were gifts from Dr Rakesh GOEL at the Ottawa Regional Cancer Center, Ottawa, Canada. These cell lines were cultured in RPMI-1640 containing 10% FBS. The paclitaxel-resistant ovarian carcinoma cell line A2780/Taxol was cultured in RPMI-1640 containing 10% FBS and 80 nmol/L paclitaxel. The human epithelial ovarian adenocarcinoma cell lines SKOV3 and CAOV3 were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and were cultured in DMEM containing 10% FBS. All the cells were cultured in a humidified incubator with 5% CO2 at 37 °C.
Small interfering RNA transfection
The oligonucleotides that comprise the double-stranded small interfering RNA (siRNA) targeting Hec1 (S: 5′-AAGUUCAAAAGCUGGAUGAUCUU-3′; AS: 5′-AAGAUCAUCCAGCUUUUGAACU-3′), which target position 1517-1539 relative to the start codon (accession number NM_006101)6, were purchased from Invitrogen (USA). According to the manufacturer's instruction, the siRNA was transfected into the ovarian cancer cells using Lipofectamine™ 2000 (Invitrogen, USA). An siRNA targeting Green Fluoresce Protein (GFP) was utilized as a control (S: 5′-AAGAAGAAGTCGTGCTGCTTCCCTGTCTC-3′; AS: 5′-AA GAAGCAGCACGACTTCTTC CCTGTCTC-3′).
RNA extraction and semi-quantitative real-time PCR
Total RNA was isolated from each group of cells using Trizol according to the manufacturer's protocol. DNase I (Promega) was included to decrease genomic DNA contamination. The reactions were performed in a Bio-Rad system using the Real-time PCR Syber Mix (DBI). The primers used in the real-time PCR reaction were as follows: Hec1: forward, 5′-GCAAGCTTCAGATACTTGCACGGTTTAC-3′, reverse, 5′-GCCTCGAGATCAACATTTTATCTGCATTCT-3′; GAPDH: forward, 5′-TGCACCACCAACTGCTTAGC-3′, reverse, 5′-GGCATGGACTGTGGTCATGAG-3′11.
After a pre-denaturation step at 95 °C for 3 min, 40 cycles of PCR were performed as follows: 15 s denaturation at 95 °C, 15 s annealing at 60 °C and a 30 s extension at 72 °C. The fold amplification for each gene was calculated using the 2−ΔΔCt method.
Western blot analysis
The cells were lysed in RIPA Lysis Buffer (Beyotime, China), and the protein concentrations were determined. Approximately 50 μg of protein was separated on a 10% SDS-polyacrylamide gel, transferred to a PVDF membrane, and incubated with antibodies against Hec1 (ab3613, Abcam, 1:1000), H3P (Ser10, e173, Epitomics, 1:1000), CYCLIN B1, Bax, cleaved PARP (4135S, 5023, 9541, Cell Signaling, 1:1000), Bcl-xL (AB126, Beyotime, 1:500), and Actin (AA128, Beyotime, 1:1000). Specific signals were detected using the alkaline phosphatase method.
MTT assay
The cells were seeded into 96-well culture plates. After a series of treatments, the cells were exposed to different doses of paclitaxel (T1912, Sigma) and incubated for 72 h. The cells were assayed by adding 100 μL/well of medium containing 1 mg/mL 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT, Sigma) for 4 h at 37 °C in a CO2 incubator. After the incubation, the MTT solution was removed, and the cells were resuspended in 200 μL of 100% dimethyl sulfoxide (DMSO). The absorbance values were measured at 570 nm and were normalized to the corresponding control (paclitaxel-untreated cells). The experiments were conducted in triplicate.
Apoptosis analysis by flow cytometry
The cells were harvested in 0.25% trypsin and washed once with PBS. After centrifugation, the cells were stained using the Annexin V-FITC/PI Apoptosis Detection Kit (KeyGen Biotech). The analysis of the apoptotic cells was performed on the flow cytometer (BD).
Cell cycle analysis by flow cytometry
The cells were harvested in 0.25% trypsin and washed once with PBS. After centrifugation, the cells were fixed in 70% ice-cold ethanol overnight at −20 °C. After a PBS wash, the cells were incubated in 500 μL sample buffer containing 50 mg/L propidium iodide (PI) and 100 mg/L RNase for 30 min. The analysis of the apoptotic cells was performed on the flow cytometer.
Immunofluorescent microscopy
Following the treatment, the cells were fixed with cold ethanol for 10 min. After an incubation with 5% BSA, the cell membranes were ruptured with 0.1% Triton X-100 for 15 min. The mouse anti-Hec1 antibody (1:50, Abcam) was incubated with the cells overnight at 4 °C. After the incubation with the appropriate secondary fluorescent antibody, the cells were stained with Hoechst-33342 and immediately observed under a fluorescent microscope.
Tissue samples and immunohistochemistry
Six normal ovarian tissues and 30 ovarian cancer tissues were collected from the Department of Pathology at Tongji Hospital after receiving approval from the Institutional Review Board. The staging was based on the FIGO classification; 12 stage I/II cases and 18 stage III/IV cases were included. The degree of cell differentiation was as follows: 9 cases were well differentiated, 13 were moderately differentiated, and 8 were poorly differentiated. A total of 20 patients with advanced-stage ovarian carcinoma that had received adjuvant paclitaxel-based chemotherapy were enrolled. Of these patients, the 8 that experienced disease progression or recurrence <6 months after discontinuing chemotherapy comprised the paclitaxel-resistant group, and the 12 that did not have a recurrence or had a disease recurrence more than 6 months after discontinuing chemotherapy comprised the paclitaxel-sensitive group. After removing the paraffin and dehydrating the ovarian tissue sections (5 μm thick), citrate buffer was utilized to unmask the antigenic sites (antigen retrieval), and the standard Avidin-Biotin Complex (ABC) method was performed to stain the sections. The positive staining was developed using DAB peroxidase substrate solution. A mouse anti-Hec1 (Abcam, 1:100) primary antibody was used.
Scoring the immunohistochemical staining
The immunohistochemical staining was scored based on both the percentage of positive DAB staining and the staining intensity12. The following scoring metric for the DAB staining intensity was utilized: 0 for negative, 1 for weak light yellow staining, 2 for medium positive yellow staining, and 3 for strong positive brown staining. The DAB staining was scored based on the percentage of the total area that was positive. The combined weighted score comprised the proportion of DAB staining for each score. For example, a case with 55% staining with a score of 3, 10% with a score of 2, and 35% with a score of 1 would be tallied as (55×3)+(10×2)+(35×1)=220. The maximum score was 300.
Statistical analysis
The SPSS16.0 software package was used to analyze the data. Statistical significance was defined as P<0.05. All the values are presented as the mean±standard deviation (SD). All the experiments were repeated at least three times.
Results
The overexpression of Hec1 protein in human ovarian cancer
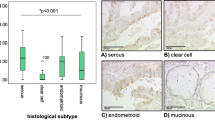
Six normal ovarian tissues and 30 ovarian cancer tissues were collected, and Hec1 expression was determined by immunohistochemistry. The results revealed that Hec1 expression was higher in ovarian cancer than in normal ovarian tissue (Figure 1A). Using the scoring criteria for the DAB staining that was described in the methods section, the mean score for the Hec1 expression in ovarian cancer was 205±47, which was higher than the expression in normal tissue (132±34) (Figure 1B, P<0.05). To investigate the clinical significance of the expression of Hec1 in ovarian cancer tissues, we analyzed the relationship between the Hec1 IHC score and the clinical drug resistance and patient prognosis. The Hec1 immunohistochemistry scores strongly correlated with paclitaxel resistance: the paclitaxel-resistant group score was 235±35 and the paclitaxel-sensitive group score was 186±33 (Figure 1C, P<0.01). Therefore, Hec1 may play an important role in ovarian cancer progression and paclitaxel resistance.
Hec1 is overexpressed in human ovarian cancer. (A) Hec1 exhibited weak staining in normal tissue (left) and strong positive expression in ovarian cancer (right) (DAB stain, ×200). (B) The immunohistochemical staining scores are illustrated in the scatterplot. The mean score for Hec1 expression in cancer was higher than that in normal tissue (the mean score is illustrated by the red line, P<0.05). (C) The relationship between the Hec1 IHC score and paclitaxel-resistance is depicted. The mean score for Hec1 expression was higher in paclitaxel-resistant cancers than in the paclitaxel-sensitive group (the mean score is depicted by the red line, P<0.05).
The mRNA and protein levels of Hec1 in various ovarian cancer cell lines
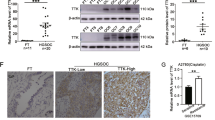
We confirmed that Hec1 was overexpressed in ovarian cancer tissues. Next, we determined whether Hec1 expression differed in various ovarian cancer cell lines (including A2780, A2780/Taxol, C13K, SKOV3, CaOV3, and ov2008). At the mRNA level, Hec1 was most highly expressed in A2780/Taxol cells, and the lowest expression was observed in A2780 cells (Figure 2A). Moreover, the same results were obtained by examining the protein expression of Hec1 by Western blot (Figure 2B). The results from the cells and the clinical samples were consistent. Hec1 is important for mitosis; its depletion impairs chromosome congression and leads to persistent activation of the spindle checkpoint. Therefore, Hec1 may play a role in the resistance to paclitaxel in ovarian cancer.
mRNA and protein expression of Hec1 in various ovarian cancer cell lines. (A) Expression of Hec1 in 6 ovarian cancer cell lines [C13K, ov2008, CaOV3, SKOV3, A2780, and A2780/Taxol (A2780T)] was determined by real-time PCR. Hec1 expression was higher in the A2780/Taxol cell line than in others; it was especially higher than for A2780 cells. The relative expression was calculated using the 2−ΔΔCt method and was compared with the A2780 cells. (B) Results from the Western blot analysis of Hec1 protein expression in the 6 cell lines demonstrated the same expression differences that were observed by real-time PCR. In each lane, 50 μg total protein was analyzed.
Depleting Hec1 expression in the ovarian cancer cell lines A2780 and A2780/Taxol
To determine whether Hec1 can improve paclitaxel resistance in ovarian cancer, we utilized siRNA targeting the coding region of Hec1 to deplete expression of Hec1 in the A2780 and A2780/Taxol paired cell lines. We used Western blotting and real-time PCR to evaluate the efficiency of siRNA in silencing expression of Hec1. Real-time PCR (Figure 3A) and Western blot (Figure 3B) results demonstrated that Hec1 expression was decreased in siRNA-transfected cell lines compared with cell lines that did not receive siRNA and those transfected with control siRNA. We also performed immunofluorescence to detect Hec1 expression at the kinetochores (Figure 3C). Compared with control cells, fluorescence intensity in Hec1 siRNA-treated cells was visibly diminished. Therefore, the Hec1 siRNA was suitable for subsequent studies.
Hec1 was depleted in the A2780 and A2780/Taxol ovarian cancer cell lines. The expression of Hec1 was decreased by specific Hec1-targeting siRNA. Forty-eight hours after the transfection of 100 nmol/L Hec1 siRNA or control siRNA, the expression of Hec1 in A2780 and A2780/Taxol cells was determined by real-time PCR (A) and Western blot(B). siRNA-transfected cells were stained with DAPI (blue) and an antibody to Hec1 (red) (C). The scale bar represents 5 μm.
Enhancing paclitaxel sensitivity by depleting Hec1
Compared with the A2780 cell line, the A2780/Taxol cell line was more resistant to paclitaxel. Previously, we demonstrated that expression of Hec1 is higher in A2780/Taxol cells than in A2780 cells. To determine whether depleting the expression of Hec1 would alter the efficacy of paclitaxel, we examined the synergism between Hec1 siRNA and paclitaxel in these two cell lines. First, we determined the effect of Hec1 knockdown on cell proliferation in the absence of paclitaxel because the Hec1 siRNA itself exhibited cytotoxicity. The results revealed that there was a difference in cell proliferation between the cells treated with 40 nmol/L Hec1 siRNA and those receiving control siRNA, but the inhibition in the A2780/Taxol cells was not as pronounced as in the A2780 cells (Figure 4A). Second, we examined the cytotoxicity of paclitaxel by transfecting cells with either Hec1 siRNA or GFP siRNA; 24 h later, we treated the cells with various concentrations of paclitaxel. After 72 h, an MTT assay was performed, and the results demonstrated that cell viability significantly decreased when Hec1 was depleted from A2780/Taxol cells (Figure 4B). The combination treatment reduced the IC50 of paclitaxel from 590±26.7 nmol/L to 45.6±19.4 nmol/L in A2780/Taxol cells, a nearly 10-fold decrease. No effects were observed in A2780 cells.
Sensitivity to paclitaxel was enhanced by depleting Hec1 in A2780 and A2780/Taxol cells. (A) A MTT assay detected the inhibition of cell proliferation after transfecting cells with control siRNA or Hec1 siRNA. (B) Twenty-four hours after the siRNA transfection, the cells were treated with a concentration gradient of paclitaxel. After 72 h, the cell viability was measured by MTT. Mean±SD. n=3. bP<0.05.
Effects of Hec1 siRNA on paclitaxel-induced apoptosis and cell cycle perturbations in A2780/Taxol cells
To confirm that depleting Hec1 reverses paclitaxel resistance, the effects of the combination treatment on apoptosis and mitotic arrest were examined by flow cytometric (FCM) analyses. The results revealed that the apoptosis rate was 9.5% in A2780/Taxol cells treated with 100 nmol/L paclitaxel versus 18% when these cells were depleted of Hec1 (Figures 5A and 5B). In the co-treated cells, the apoptosis rate was 35%, a nearly 2.5-fold increase compared with paclitaxel treatment and a 1.0-fold increase compared with Hec1 siRNA treatment. More co-treated cells were arrested in G2/M compared with cells receiving a single treatment (Figure 5C). Therefore, G2/M arrest might prolong paclitaxel activity, which could reduce paclitaxel resistance.
Effects of Hec1 siRNA on paclitaxel-induced apoptosis and cell cycle perturbations in A2780/Taxol cells. Forty-eight hours after the siRNA transfection, the cells were treated with 100 nmol/L paclitaxel. After 24 h, the cells were analyzed by flow cytometry. (A) The cells were stained with Annexin-V FITC and propidium iodide after various treatments to identify those undergoing apoptosis. (B) The percentage of apoptotic cells in response to various treatments is presented. (C) Following treatment, the cells were harvested, fixed and stained with propidium iodide. After various treatments, the percentage of A2780/Taxol cells in each phase of the cell cycle was determined by flow cytometry. Mean±SD. n=3. bP<0.05.
Effects of Hec1 siRNA on expression of cell cycle- and apoptosis-related proteins in A2780/Taxol cells
By flow cytometry, the depletion of Hec1 enhanced mitotic arrest and paclitaxel-induced apoptosis. Therefore, to explore the role of Hec1 in improving sensitivity to paclitaxel, we performed Western blots to ascertain changes in expression levels of cell cycle- and apoptosis-related proteins (Figure 6). A2780/Taxol cells were treated with paclitaxel, Hec1 siRNA, or both factors. Cleaved PARP was up-regulated when cells were co-treated with Hec1 siRNA and paclitaxel. Expression of proteins that mediate cell death, such as Bax, was increased, but anti-apoptotic proteins, such as Bcl-xL, were significantly downregulated. These paclitaxel chemotherapies might be more effective in combination with Hec1 knockdown. Cyclin B1, which is up-regulated in G2/M, and H3P (phospho-histone H3), which is tightly correlated with chromosome condensation during both mitosis and meiosis via phosphorylation at Ser10, were both up-regulated by the combination treatment. All the results indicated that the Hec1 siRNA/paclitaxel combination treatment enhanced mitotic arrest and paclitaxel-induced apoptosis.
Effects of Hec1 siRNA on the expression of cell cycle- and apoptosis-related proteins in A2780/Taxol cells. Forty-eight hours after the siRNA transfection, the cells were treated with 100 nmol/L paclitaxel. After 24 h, the cells were analyzed by Western blot to ascertain changes in expression levels of cell cycle- and apoptosis-related proteins. (A) Representative images of the Western blot analysis for expression level of cell cycle- and apoptotisis-related proteins. (B) Bar graph for the quantitative comparison among protein expression levels. The signal intensities of a protein band and its surrounding background were quantified by using Gel-pro analysis 4. The resultant background-subtracted values of protein expression were normalized to those of actin and then plotted as the relative protein levels for each protein. Mean±SD. n=3. bP<0.05, cP<0.01.
Discussion
Ovarian cancer is the most common cause of death from a gynecological malignancy in the world. Although chemotherapy remains the primary treatment for human ovarian cancer, chemoresistance is a clinical problem that severely limits treatment efficacy. Therefore, understanding the mechanisms that contribute to drug resistance will provide knowledge with which to develop more effective anti-cancer chemotherapies. Paclitaxel is a first-line treatment for ovarian cancer chemotherapy. Drug resistance is the primary cause of treatment failure in patients with cancer.
Paclitaxel binds to tubulin and promotes the formation of stable microtubules. Paclitaxel also impedes the cell cycle by causing cells to accumulate in M phase, eventually leading to apoptosis. The mechanisms responsible for paclitaxel resistance include the following: 1) changes in the expression level of her/neu and Bcl-213; 2) increased microtubule dynamics14; 3) damage of the mitotic spindle checkpoint3; and 4) chromosomal instability15,16. Swanton and colleagues have identified numerous genes responsible for paclitaxel resistance, most of which are important for mitosis. When they inhibited the expression of these mitosis-related genes, paclitaxel resistance increased because of damage to the mitotic spindle checkpoint17. Numerous genes have been targeted as chemotherapeutics or to improve the effects of chemotherapy. For example, inhibition of AURKA expression suppressed tumor growth and enhanced the chemosensitivity to taxane18. Chromosomal instability has been suggested to be a driving force for tumor initiation and/or progression. Many genes known to play a role in the proper control of mitosis are mis-regulated in tumors. Particular mitotic regulatory genes involved in chromosome segregation, such as Hec1 and Mad2, are highly expressed in various human tumors and are correlated with tumor grade and prognosis19,20. Here, we report similar results from immunohistochemical studies on human ovarian cancer: Hec1 was overexpressed in ovarian cancer, with the highest expression in paclitaxel-resistant ovarian cancer tissues and A2780/Taxol cells. Moreover, Hec1, an important mitotic protein, is intricately involved in establishing appropriate microtubule attachments and chromosome alignment21. Therefore, the results suggested that Hec1 might be an effective indicator for improving paclitaxel resistance in ovarian cancer. Ultimately, when we used siRNA to diminish Hec1 expression in A2780/Taxol cells, progression through the M phase was inhibited and paclitaxel-induced apoptosis was increased.
Mitotic arrest is necessary for the cytotoxicity of these agents because they induce mitotic cell death, demonstrating that prolonged mitotic checkpoint activation is important for the formation and treatment of cancer22. Many researchers have suggested that frequent inactivation of the mitotic spindle checkpoint in cancer might contribute to the resistance of tumor cells to chemotherapeutics23. Because elevated Hec1 expression was observed in various cancers, multiple studies have endeavored to identify the effects on tumor therapy and chemoresistance. It has been reported that Hec1 is an important prognostic marker in breast cancer. A small molecule-induced decrease in Hec1 expression conspicuously inhibited tumor growth10. In our experiments, Hec1 knockdown recovered spindle checkpoint activity, induced accumulation of cells in G2/M, and prolonged mitotic arrest, similar to what has previously been shown with the inhibition of AURKA expression. Gascoigne and Taylor proposed an elegant “competing-networks” model for cell fate in the presence of spindle poisons24. In their model, mitotic slippage occurs when cyclin B drops below a critical threshold, whereas cell death occurs when the apoptosis-activating network reaches a critical threshold. Prolonging mitotic arrest may provide sufficient time to activate apoptosis. The final result was an enhancement of paclitaxel-induced apoptosis and an improvement in paclitaxel sensitivity. Although the precise molecular mechanism remains to be explored, the inhibition of Hec1 may be an effective approach for cancer therapy.
Author contribution
Qing-qing MO performed the immunohistological staining, detected the expression of Hec1 in the cell lines, analyzed in changes in cell viability after treatment with Hec1 siRNA, performed the apoptosis and cell cycle assays and drafted the manuscript. Ping-bo CHEN designed the study and supported the data analyses. Xin JIN supported the molecular studies. Qian CHEN and Ke-zhen LI helped Qing-qing MO with the immunohistochemical staining and analyzed the immunohistochemical data. Lan TANG helped Qing-qing MO with the flow cytometry experiments. Bei-bei WANG, Peng WU, and Yong FANG participated in the design of the study and provided expertise on molecular biological techniques. Shi-xuan WANG, Jian-feng ZHOU, and Ding MA participated in the writing of the manuscript. Gang CHEN participated in the design of the study and critically revised the manuscript.
References
Wang TH, Wang HS, Soong YK . Paclitaxel-induced cell death: where the cell cycle and apoptosis come together. Cancer 2000; 88: 2619–28.
Anand S, Penrhyn-Lowe S, Venkitaraman AR . AURORA — A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell 2003; 3: 51–62.
Masuda A, Maeno K, Nakagawa T, Saito H, Takahashi T . Association between mitotic spindle checkpoint impairment and susceptibility to the induction of apoptosis by anti-microtubule agents in human lung cancers. Am J Pathol 2003; 163: 1109–16.
Sudo T, Nitta M, Saya H, Ueno NT . Dependence of paclitaxel sensitivity on a functional spindle assembly checkpoint. Cancer Res 2004; 64: 2502–8.
DeLuca JG, Howell BJ, Canman JC, Hickey JM, Fang G, Salmon ED . Nuf2 and Hec1 are required for retention of the checkpoint proteins Mad1 and Mad2 to kinetochores. Curr Biol 2003; 13: 2103–9.
Martin-Lluesma S, Stucke VM, Nigg EA . Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science 2002; 297: 2267–70.
Wei R, Ngo B, Wu G, Lee WH . Phosphorylation of the Ndc80 complex protein, HEC1, by Nek2 kinase modulates chromosome alignment and signaling of the spindle assembly checkpoint. Mol Biol Cell 2011; 22: 3584–94.
Glinsky GV, Berezovska O, Glinskii AB . Microarray analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. J Clin Invest 2005; 115: 1503–21.
van't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002; 415: 530–6.
Wu G, Qiu XL, Zhou L, Zhu J, Chamberlin R, Lau J, et al. Small molecule targeting the Hec1/Nek2 mitotic pathway suppresses tumor cell growth in culture and in animal. Cancer Res 2008; 68: 8393–9.
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 2002; 3: RESEARCH0034.
Ashida S, Furihata M, Katagiri T, Tamura K, Anazawa Y, Yoshioka H, et al. Expression of novel molecules, MICAL2-PV (MICAL2 prostate cancer variants), increases with high Gleason score and prostate cancer progression. Clin Cancer Res 2006; 12: 2767–73.
Perez-Soler R, Kemp B, Wu QP, Mao L, Gomez J, Zeleniuch-Jacquotte A, et al. Response and determinants of sensitivity to paclitaxel in human non-small cell lung cancer tumors heterotransplanted in nude mice. Clin Cancer Res 2000; 6: 4932–8.
Goncalves A, Braguer D, Kamath K, Martello L, Briand C, Horwitz S, et al. Resistance to Taxol in lung cancer cells associated with increased microtubule dynamics. Proc Natl Acad Sci U S A 2001; 98: 11737–42.
Swanton C, Nicke B, Schuett M, Eklund AC, Ng C, Li Q, et al. Chromosomal instability determines taxane response. Proc Natl Acad Sci U S A 2009; 106: 8671–6.
McClelland SE, Burrell RA, Swanton C . Chromosomal instability: a composite phenotype that influences sensitivity to chemotherapy. Cell Cycle 2009; 8: 3262–6.
Swanton C, Marani M, Pardo O, Warne PH, Kelly G, Sahai E, et al. Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer Cell 2007; 11: 498–512.
Hata T, Furukawa T, Sunamura M, Egawa S, Motoi F, Ohmura N, et al. RNA interference targeting aurora kinase a suppresses tumor growth and enhances the taxane chemosensitivity in human pancreatic cancer cells. Cancer Res 2005; 65: 2899–905.
Perez de Castro I, de Carcer G, Malumbres M . A census of mitotic cancer genes: new insights into tumor cell biology and cancer therapy. Carcinogenesis 2007; 28: 899–912.
Garber ME, Troyanskaya OG, Schluens K, Petersen S, Thaesler Z, Pacyna-Gengelbach M, et al. Diversity of gene expression in adenocarcinoma of the lung. Proc Natl Acad Sci U S A 2001; 98: 13784–9.
DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED . Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell 2006; 127: 969–82.
Weaver BA, Cleveland DW . Decoding the links between mitosis, cancer, and chemotherapy: The mitotic checkpoint, adaptation, and cell death. Cancer Cell 2005; 8: 7–12.
Wang L, Yin F, Du Y, Chen B, Liang S, Zhang Y, et al. Depression of MAD2 inhibits apoptosis and increases proliferation and multidrug resistance in gastric cancer cells by regulating the activation of phosphorylated survivin. Tumour Biol 2010; 31: 225–32.
Gascoigne KE, Taylor SS . How do anti-mitotic drugs kill cancer cells? J Cell Sci 2009; 122: 2579–85.
Acknowledgements
This project was supported by National Natural Science Foundation of China (81272859; 81230038; 81101962; 81025011; 81101962; 81071663); Nature and Science Foundation of Hubei Province (2011CBD542) and Grants from the Fundamental Research Funds for the HUST(2012TS058).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mo, Qq., Chen, Pb., Jin, X. et al. Inhibition of Hec1 expression enhances the sensitivity of human ovarian cancer cells to paclitaxel. Acta Pharmacol Sin 34, 541–548 (2013). https://doi.org/10.1038/aps.2012.197
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2012.197