Abstract
In recent decades, the pharmaceutical application potential of marine natural products has attracted much interest from both natural product chemists and pharmacologists. Our group has long been engaged in the search for bioactive natural products from Chinese marine flora (such as mangroves and algae) and fauna (including sponges, soft corals, and mollusks), resulting in the isolation and characterization of numerous novel secondary metabolites spanning a wide range of structural classes and various biosynthetic origins. Of particular interest is the fact that many of these compounds show promising biological activities, including cytotoxic, antibacterial, and enzyme inhibitory effects. By describing representative studies, this review presents a comprehensive summary regarding the achievements and progress made by our group in the past decade. Several interesting examples are discussed in detail.
Similar content being viewed by others
Introduction
The unique ocean habitat has caused marine organisms to evolve distinctive metabolic pathways, producing remarkable secondary metabolites that differ from those of terrestrial plants. Because the compounds isolated from marine organisms are structurally and biologically intriguing, research on marine natural products has been attracting the attention of chemists as a challenging research topic. To date, more than 30 000 compounds bearing unusual structures and exhibiting various bioactivities have been isolated from marine plants and invertebrates.
In recent years, the interest in natural products from Chinese marine organisms has increased dramatically, making the annual average number of natural products isolated from marine organisms increase rapidly. As one of the most active research groups in this area, we have been devoted to the investigation of Chinese marine plants and invertebrates since 2000. In the course of our studies, a series of structurally diverse and biologically interesting compounds belonging to various structural classes, such as macrocyclic polydisulfides, spirodioxynaphthalene, and alkaloids, have been isolated and characterized. Through these productive studies, we have made important contributions to the research and development of Chinese marine natural products. This review summarizes the progress and achievements made by our group in the study of Chinese marine flora and fauna in the past decade, and several interesting examples are discussed in detail.
Chemical studies on Chinese marine plants
Algae
Microalgae play important roles in marine biological systems. Through their photosynthetic ability, algae are the major producers of biomass and organic compounds in the ocean. More importantly, many algal metabolites present unique structures and are formed via biosynthetic routes that are quite different from those known to produce terrestrial metabolites.
Marine dinoflagellates are flagellated organisms with both photosynthetic and heterotrophic attributes. The lifestyle of these organisms is diverse, and many of them participate in symbiotic relationships. For some time, the study of dinoflagellates has focused on their toxin production and pigment composition, and only a small number of these organisms have been found to produce other secondary metabolites. As part of our ongoing research on the biologically active substances of Chinese marine organisms, three new polyhydroxylpolyene compounds, lingshuiols A and B (1 and 2) and lingshuiol (3), have been isolated from the Chinese marine dinoflagellate Amphidinium sp collected at Lingshui Bay, Hainan Province, China1,2 (Figure 1). The structures of these compounds are characterized by linear carbon chains, as determined by extensive analyses of 2D NMR spectroscopic data; however, the absolute configurations remain to be determined. In previous studies, polyhydroxylpolyene compounds were found to exhibit antifungal, hemolytic, and antimicrobial activities3,4,5. It was demonstrated that lingshuiol (3) possessed potent in vitro cytotoxic activities against A549 and HL-60 cells, with IC50 values of 0.21 and 0.23 μmol/L, respectively.
Red algae of the genus Laurencia are found throughout the world, mostly in tropical and subtropical regions. Since Irie's pioneering investigations on Laurencia over the last four decades6, a number of cuparene- and laurane-derived sesquiterpenoids have been isolated from this genus. The chemical investigation of the Chinese red alga Laurencia okamurai Yamada led to the isolation of a new laurane sesquiterpenoid, 3β-hydroxyaplysin (4), and two novel rearranged sesquiterpenes, laurokamurenes A and B (5 and 6)7 (Figure 2). Their structures, including their relative stereochemistry, were determined based on a detailed interpretation of 2D NMR spectra and comparisons with related known compounds. To the best of our knowledge, this is also the first report of the isolation of sesquiterpenes containing a 2,2,3-trimethylcyclopentenyl moiety from a natural source. The sesquiterpenoids isolated from the red algae of the genus Laurencia in recent years can be classified into more than 20 sesquiterpenoid skeletons8. However, in most cases, the three methyl groups in the aliphatic portion are located at either positions 1,2,3 (laurene type) or 1,2,2 (cuparene type), with the exception of laurokamurenes A and B (5 and 6).
Two novel aromatic valerenane-type sesquiterpenes, caulerpals A and B (7 and 8), were isolated from the Chinese green alga Caulerpa taxifolia9 (Figure 2). Compounds 7 and 8 were evaluated for their inhibitory activity against human protein tyrosine phosphatase 1B (PTP1B), a potential drug target for the treatment of type 2 diabetes and obesity, but neither compound exhibited a potent PTP1B inhibitory activity. It is important to note that caulerpals A and B (7 and 8) are the only two known compounds with an aromatic valerenane-type carbon skeleton from a natural source.
Mangroves
Mangroves comprise a large number of various salt-tolerant plants growing in tropical and subtropical intertidal estuarine zones. Mangrove plants are usually categorized into two subgroups, true mangrove and semi-mangrove plants, according to their living environment. True mangrove plants are confined to the typical intertidal mangrove habitats where the seawater salinity is usually 17.0‰–36.4‰. Semi-mangrove plants, however, grow on the landward fringe of the mangrove habitats or in the terrestrial marginal zones that are subjected to irregularly high tides. In general, mangrove plants consist of 84 species globally, belonging to 24 genera and 16 families; 14 are semi-mangrove species10,11.
Most of our studies involve plants belonging to the Rhizophoraceae, Sonneratiaceae, Euphorbiaceae, and Meliaceae families. A series of unusual compounds bearing unprecedented structures were identified in these taxa, some of which showed marked biological activities and are currently in preclinical studies.
For instance, an unusual novel macrocyclic polydisulfide gymnorrhizol (9) was isolated from the mangrove plant Bruguiera gymnorrhiza (Rhizophoraceae family). Compound 9 possesses an uncommon carbon skeleton that is characteristic of a 15-membered macrocycle composed of 3 repeated 1,3-dimercaptopropan-2-ol units12 (Figure 3). The structure of compound 9 was established by extensive spectroscopic studies and confirmed using X-ray crystallography13. The first total synthesis of gymnorrhizol (9) was completed by our group with a high overall yield14 (Scheme 1).
Prompted by the unusual structure of gymnorrhizol (9) and its potent inhibitory activity against PTP1B (an IC50 value of 14.9 μmol/L), we investigated the same mangrove species collected from different areas, which led to the isolation of two new macrocyclic polydisulfides, 10 and 1115. The structures of 10 and 11 were unequivocally determined through single-crystal X-ray diffraction analysis.
A plausible biosynthetic pathway for these cyclic polydisulfides was also proposed in which the 1,3-dimercaptopropan-2-ol unit should be the common building block for the biosynthesis of all of these macrocyclic polydisulfides; the self-cyclization of the 1,3-dimercaptopropan-2-ol unit of different sizes gave rise to macrocyclic polydisulfides ranging from 5-membered to 30-membered polydisulfides or even to larger polydisulfides. Marine disulfide- and multisulfide-containing metabolites are a special and important class of natural products. Due to the increasing interest in these compounds, the progress in this area was previously summarized by our group16, encompassing the literature published between 1971 and 2010.
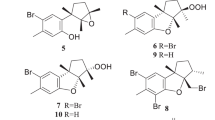
To obtain sufficient amounts of the polydisulfides for pharmacological research, the mangrove plant B gymnorrhiza collected from Zhanjiang, Guangdong Province, was investigated. Unexpectedly, instead of the ubiquitous macrocyclic polydisulfides, seven new spirodioxynaphthalene compounds, named palmarumycins BG1–BG7 (12–18) (Figure 4), were found17. It is noteworthy that these intriguing compounds were generally considered to be fungal metabolites, even though there were four reports on the isolation of palmarumycins (or preussomerins) from plants18,19,20,21. The fact that compounds 12–18 were isolated in quite an appreciable quantity indicated that these compounds were produced by B gymnorrhiza; however, we could not rule out the possibility that they were the metabolites of an endophytic or epiphytic fungus. The absolute configurations of metabolites 12–18 were determined using time-dependent density functional theory electronic circular dichroism (TDDFTECD) calculations of the solution conformers. It was found that compound 16 displayed an inhibitory activity against the HL-60 and MCF-7 cell lines. Additionally, we published a report summarizing the literature on the isolation, structure elucidation, biological activities, biosynthesis, and chemical synthesis of spirodioxynaphthalenes over the last 20 years22.
Although the chemical investigation of the plants belonging to the Sonneratiaceae family was not as fruitful as that of the Rhizophoraceae family, a novel α-alkylbutenolide dimer, paracaseolide A (19), was isolated from the mangrove plant Sonneratia paracaseolaris (Sonneratiaceae family); this compound is characterized by an unusual tetraquinane oxa-cage bislactone skeleton bearing two linear alkyl chains23 (Figure 5). Moreover, paracaseolide A (19) exhibited a potent inhibitory activity against the dual-specificity phosphatase Cdc25B, a key enzyme for cell cycle progression, with an IC50 value of 6.44 μmol/L. A retrosynthetic pathway for paracaseolide A (19) was proposed in which 19 should be biosynthesized through a Diels-Alder [4+2] cycloaddition, starting from two molecules of 20 (Scheme 2). Paracaseolide A (19) represents the first example of an α-alkylbutenolide dimer from a natural source. Upon publication, the article was immediately covered in the journal of Natural Product Reports as “hot off the press”24.
In addition, three known compounds, integracins A and B (21 and 22) and 15-dehydroxy-integracin B (24), were isolated for the first time from the Chinese mangrove plant Sonneratia hainanensis25 (Figure 5). Although compounds 21–24 had been reported previously, their absolute configurations were not resolved until their absolute chemistry was determined using Mosher's method, the specific rotation analysis of the alcohols (24 and 25) obtained from integracin A (21) in two steps, and chemical correlation. Integracin A (21) exhibited a cytotoxic effect against the tumor cell lines HepG2 and NCI-H460, both of which were 100% inhibited at 25 mg/mL.
The Euphorbiaceae family is composed of various species that are distributed widely throughout China. Although poisonous, most of the plants in this family are utilized in folk medicine. Thus, the substances of the mangrove plant Excoecaria agallocha L (Euphorbiaceae family for the first time) were investigated, which led to the isolation of four diterpenes with an unprecedented skeleton, named excoagallochaols A–D (26–29)26 (Figure 6). In addition to these terpenes, a novel octacyclic alkaloid with an unusual scaffold, suffruticosine (30), was isolated from Securinega suffruticosa (Pall) Rehd in the same family27 (Figure 6). The absolute configuration of suffruticosine (30) was determined on the basis of CD spectral analysis.
Plants of the Meliaceae family are a rich source of limonoids, which are a structurally diverse group of natural products with highly complex polycyclic skeletons. These unusual structural features have attracted the attention of chemists as challenging targets for total synthesis, bioactivity evaluation, and biosynthetic studies. Chemical investigation on the stem bark of the plant Toona ciliata var pubescens resulted in the isolation of nine new compounds, including toonapubesin A (31), toonapubesic acid A (32), and their derivatives28 (Figure 6). The proposed structure of 32 was confirmed by the X-ray diffraction analysis of its methyl ester, whereas the absolute configuration was determined by a novel solid-state TDDFT ECD approach on its methyl ester.
More importantly, macrolide (R)-de-O-methyllasiodiplodin (33), isolated from the semi-mangrove Cerbera manghas, was found to be a potent nonsteroidal antagonist of the mineralocorticoid receptor (MR), a drug target for the treatment of hypertension and other cardiovascular diseases, showing an IC50 value of 8.93 μmol/L. Due to its low natural yield, further study on its structural derivatization/modification and the structure-activity relationship (SAR) was conducted. Consequently, a series of analogs were synthesized and evaluated for their MR antagonistic activities29, and compounds 34–36 were found to exhibit a better selectivity and more potent activity against MR than compound 33, with IC50 values ranging from 0.58 to 1.11 μmol/L.
Chemical studies on Chinese invertebrates
Sponges
Sponges are animals of the phylum Porifera, which consists of approximately 5 000–10 000 species that can be classified mainly according to the composition of their skeletons as calcarea, glass sponges, and demosponges. Their chemical variability results from the individual species, particular metabolism and complex environment. In the past half century, a large number of compounds, including terpenoids, alkaloids, and polyacetylenes, were isolated and characterized from various sponges collected from different regions of the world.
For instance, five new sesquiterpenes, lingshuiolides A and B (37 and 38), lingshuiperoxide (39), isodysetherin (40), and spirolingshuidolide (41) were isolated from the Hainan sponge Dysidea septosa30 (Figure 7). The absolute configuration of lingshuiolide B (38) was established by using a modified Mosher's method. The chemical study on another Dysidea sp led to the discovery of a new sesquiterpene quinone (42), along with a known related analog dysidine (43)31 (Figure 7). A bioassay showed that dysidine (43) had a potent PTP1B inhibitory activity, with an IC50 value of 6.70 μmol/L, whereas the new compound 42 exhibited a moderate PTP1B inhibitory activity and cytotoxicity, with IC50 values of 39.50 and 19.45 μmol/L, respectively32.
Three unprecedented diastereoisomeric dimers, cis-dimers A (44) and B (45) and trans-dimer C (46), representing bis-bisabolene skeletons, and their potential precursor dehydrotheonelline (47) have been isolated from the South China Sea sponges Axinyssa variabilis and Lipastrotethya ana33 (Figure 7). The three diastereoisomers (44–46) were possibly biosynthesized through an intermolecular [4+2] Diels-Alder cycloaddition involving two molecules of dehydrotheonelline (47). The trans- and cis-dimers were possibly formed through exo- and endo-Diels-Alder coupling, respectively (Scheme 3). Furthermore, a mixture of cis-dimers 44 and 45 and the pure trans-dimer 46 were active at 50 mg/cm2 in a feeding-deterrence test against the gold fish Carassius auratus, suggesting a possible defensive role for these compounds.
Soft corals
Soft corals from the South China Sea have been extensively studied by Chinese chemists and have yielded a plethora of steroids and terpenoids. It has been suggested that such secondary metabolites produced by soft corals are most likely involved in the defensive mechanisms of the animals; indeed, the animals appear to be relatively free from predation.
As expected, the chemical study of the soft coral Lobophytum cristatum Tixier-Durivault led to the isolation of two new prenylgermacrane-type diterpenoids, lobophytumins A and B (48 and 49), two new prenyleudesmane-type diterpenoids, lobophytumins C and D (50 and 51), and two new spatane-type diterpenoids, lobophytumins E and F (52 and 53)34 (Figure 8). Their structures, including the relative configurations, were determined via a detailed analysis of the spectroscopic data and comparison with related known compounds. In addition, the absolute configuration of lobophytumin C (50) was tentatively assigned by comparing its specific rotation with that of the closely related model compound (–)-β-selinene; in contrast, the stereochemistry of C-11 in compounds 48–49 and 52–53 remains undefined. This is the first report of spatane-type diterpenoids from a soft coral source34, and it supports Faulkner's proposal of prenylgermacrene as the precursor of many diterpenes, including quite different but biogenetically related ones. In a bioactivity evaluation, lobophytumins C and D (50 and 51) showed weak in vitro cytotoxicities against the tumor cell lines A549 and HCT-116.
Casbane diterpenes are characterized by the presence of a dimethyl-cyclopropyl moiety fused to the 14-membered ring, structures that are extremely rare in nature and marine organisms and previously found only in Sinularia microclavata35. From the Hainan soft coral Sinularia depressa, we isolated nine casbane diterpenes (54–62), either cis or trans ring junctions, including two pairs of epimers: depressin (54) and 1-epi-depressin (55) and 10-hydroxydepressin (56) and 1-epi-10-hydroxydepressin (57)36 (Figure 9). Compounds 56 and 57 played a key role in the establishment of the absolute stereochemistry, as the S configuration at C-10 of both compounds could be easily assigned using Mosher's method. Three further casbanes (59–61) displayed identical structures, with the exception of the stereochemistries at the ring junction, which were cis in 59 and trans in both 60 and 61. Compound 58 was similar to 57, except for the oxidization of the hydroxyl; nevertheless, compound 62 had many similarities with 10-hydroxydepressin (56), with the exception of an additional oxygen atom. 10-Hydroxydepressin (56) showed a cytotoxic activity against the tumor cell lines HepG2 and SW-1990, with IC50 values of 61 and 37 μmol/L, respectively, and an antimicrobial activity against Staphylococcus aureus and Escherichia coli at the concentration of 17 μmol/L.
The genus Sarcophyton is a productive source for unusual tetraterpenoids, which are constructed by coupling two cembranoid units through the Diels-Alder reaction. Five novel biscembranoids, ximaolides A–E (64–68), together with their proposed biogenetic precursor, methyl tortuosoate (63), were isolated from the Hainan soft coral Sarcophyton tortuosum37 (Figure 10). The relative stereochemistries of ximaolides A and E (64 and 68) were acquired by X-ray diffraction analysis, whereas the stereochemistries of the other compounds were suggested by both biosynthetic considerations and NOESY experiments. Furthermore, ximaolide D (67) is the first example of a biscembranoid possessing a tetrahydrofuran ring between the A and B rings.
The study of Sarcophyton lactum also led to the discovery of two unprecedented biscembranoids, bislatumlides A and B (69 and 70), for which the dienophilic double bond belonged to an α,β-conjugated-γ-lactone, along with a new cembranolide diterpene, isosarcophytonolide D (71)38 (Figure 10). Compounds 69 and 70 differ in the configuration of the double bond at C-11: E for 69 and Z for 70. However, it was proven that the isomerization of the double bond in 69 readily occurred in the presence of trace amount of acid. Compounds 69 and 70 could be biosynthesized by the Diels-Alder addition of two cembranoid units. Compound 71 and the known compound sarcophytonolide D (72) may comprise half of the biosynthetic precursors, as shown in Scheme 4.
Steroids are a large group of compounds encountered in marine organisms and are reported to display various bioactivities. For instance, a novel steroid with an uncommon 21-oic acid methyl ester moiety, designated methyl spongoate (73), was isolated from the Sanya soft coral Spongodes sp; this steroid exhibited a potent cytotoxicity against BEL-7402 tumor cells in vitro39. Later, its stereoselective synthesis was achieved in our laboratory40. Owning to its unusual structure and potent cytotoxicity, a number of analogs with different C-20 side chains were synthesized and evaluated for their cytotoxic activities, and some of the analogs were found to be more potent than compound 73 against the A549, HCT-116, HepG2, SW-1990, MCF-7, and NCI-H460 tumor cell lines. The preliminary SAR study suggested that the unsaturated carbonyl moiety, a Michael acceptor in ring A, and the C-20 side chain played important roles in the cytotoxic effect of these derivatives41 (Figure 10).
Mollusks
Mollusks are a large phylum of invertebrate animals that are highly diverse in size and anatomical structure and also in their behavior and habitat. Mollusks show such a variety in body structures that it is difficult to find definite characteristics that apply to all of the classification groups. The two most universal features are a mantle with an important cavity used for breathing and excretion and the structure of the nervous system. However, most of the animals in the Opisthobranchia family lose their mantles and require other defensive system for protection. Thus, these animals usually produce structurally diverse substances to form a chemical defensive system, representing a different source of bioactive compounds from marine organisms. As expected, a series of metabolites with complex structures and intriguing biological activities have been isolated from mollusks.
The study on the South China Sea nudibranch Tritoniopsis elegans led to the isolation of four diterpenes, tritoniopsins A-D (74–77), which display an unprecedented pyran ring in the cladiellane framework and represent a novel cladiellane-based diterpene family42 (Figure 11). These compounds were also found in the nudibranch's prey, Cladiella krempfi. The structure of tritoniopsin A (74) was elucidated by X-ray diffraction analysis, whereas the S configuration of the hydroxyl at C-6 in tritoniopsin C (76) was assigned by applying Mosher's method. Both tritoniopsins A and B (74 and 75) were discovered from T elegans and C krempfi, but, surprisingly, the major compound in this mollusk was tritoniopsin A (74). Such an uneven metabolite distribution could be explained by the fact that the mollusk either modified dietary tritoniopsin B (75) or selectively accumulated compound 74. The presence of these unique metabolites in both the nudibranch and the soft coral C krempfi also confirmed the trophic relationship between the predator and prey.
During the investigation of the nudibranch Glossodoris rufomarginata and its unidentified prey sponge from the South China Sea, it was observed that scalaradial (78) and its deacetyl derivative (79) were the main components of the extract of the dietary sponge, whereas a series of related scalaranes (80–84) (Figure 11), most likely derived from dietary scalaradial, were isolated from the mollusk, thus proving the ability of Glossodoris rufomarginata to modify dietary molecules43. The finding of these molecules (80, 81, and 84) with a keto functionalization at C-12 is particularly interesting. This structural feature is quite common in the homoscalarane series but unusual in the scalaranes, and in particular it has been encountered only in nudibranch metabolites. In fact, in addition to 12-deacetoxy-12-oxo-deoxoscalarin (80), previously isolated from Indian Glossodoris atromarginata44, only one 12-keto-scalarane, 12-deacetoxy-12-oxo-18-epi-scalaradial, from Pacific nudibranch Chromodoris youngbleuthi45, has been previously reported.
In our research on the sea hare Aplysia dactylomela, two novel triterpenes, aplysiols A and B (85 and 86), characterized as squalene-derived polyethers, were isolated46 (Figure 11). The absolute stereochemistry of aplysiol A (85) was determined using Mosher's method and biogenetic considerations, whereas the configuration of aplysiol B (86) was established by an integrated NMR-QM (quantum mechanical) approach on the basis of 13C NMR chemical shifts and 2,3JC–H coupling-constant DFT (density functional theory) calculations.
Marine pulmonata of the Onchidiidae family are shell-less mollusks living on sheltered intertidal shores. Previous chemical studies on distinct species in this family mostly focused on polypropionates. In our study, onchidione (87), as the main metabolite, was characterized from the first collection of the Chinese marine pulmonate Onchidium sp (Figure 11), which was present both in the mucus and in the mantle47. The structure of the polypropionate was suggested by an analysis of spectral data. In particular, the relative stereochemistry of the central ring was supported by a series of nuclear overhauser enhancement (NOE) experiments, and the complete structure was confirmed by X-ray diffraction analysis. A recent study on the second collection of the mollusk led to the isolation of onchidiol (88) and 4-epi-onchidiol (89), related alcohols of onchidione (87)48 (Figure 11). The absolute configurations of onchidiol (88) and 4-epi-onchidiol (89) were established by TDDFTECD. Moreover, the absolute chemistry of onchidione (87) was corroborated by X-ray diffraction analysis, with the final refinement using Cu-Kα data.
In the final section of this review, we describe the nitrogen-containing compounds of marine mollusks. The first example consists of phidianidines A and B (90 and 91) obtained from Phidiana militaris, belonging to the Glaucidae family49 (Figure 11). The two novel alkaloids were characterized with an unprecedented carbon skeleton featuring the presence of a 1,2,4-oxadiazole ring linking the indole system through a methylene bridge and displaying an aminoalkylguanidine group at C-3′. The 1,2,4-oxadiazole ring is extremely rare in nature. The phidianidines were tested in a panel of tumor cell lines (C6 rat glioma cells, HeLa human epithelial cervical cancer cells and CaCo-2 human epithelial colorectal adenocarcinoma cells) and non-tumor cell lines (H9c2 rat embryonic cardiac myoblasts and 3T3-L1 murine embryonic fibroblasts) and showed a strong and selective activity against C6 and 3T3-L1 cells by IC50 values within the nanomolar range, as shown in Table 1.
The second example is an unusual molecule, actisonitrile (92), found in the mantle of Actinocyclus papillatus50 (Figure 11), belonging to the Actinocyclidae family that is another family of opisthobranch, which was never studied thus far. Despite its structure is quite simple, the determination of the absolute stereochemistry of 92 was a challenging task, yet its absolute configuration was recently solved by a synthetic approach. Thus, both the S and R enantiomers were synthesized, and the comparison of optical rotation and CD profile of the natural product with those of the pair of the synthetic enantiomers resulted in the assignment of the R configuration at the C-2 position of compound 92.
Conclusions
This review summarizes representative substances from Chinese marine plants and invertebrates found by our group, indicating that marine organisms are an inexhaustible source of new molecules that often display unique structures and sometimes have very interesting pharmacological properties, such as antifungal, antibacterial, enzyme-inhibitory, and other activities. Some of the compounds isolated from marine organisms even show promising activities within the nanomolar range and can be developed as drug candidates or at least lead compounds in the near future. Although our research on marine natural products is limited, it has established the foundation for further studies.
Because our understanding of the biosynthetic origin and the real ecological role of these bioactive compounds remains incomplete, further research to clarify these important issues is necessary.
References
Huang XC, Zhao D, Guo YW, Wu HM, Trivellone E, Cimino G . Lingshuiols A and B, two new polyhydroxy compounds from the Chinese marine dinoflagellate Amphidinium sp. Tetrahedron Lett 2004; 45: 5501–4.
Huang XC, Zhao D, Guo YW, Wu HM, Lin LP, Wang ZH, et al. Lingshuiol, a novel polyhydroxyl compound with strongly cytotoxic activity from the marine dinoflagellate Amphidinium sp. Bioorg Med Chem Lett 2004; 14: 3117–20.
Doi Y, Ishibashi M, Nakamichi H, Kosaka T, Ishikawa T, Kobayashi J . Luteophanol A, a new polyhydroxyl compound from symbiotic marine dinoflagellate Amphidinium sp. J Org Chem 1997; 62: 3820–3.
Paul GK, Matsumori N, Murata M, Tachibana K . Isolation and chemical structure of amphidinol 2, a potent hemolytic compound from marine dinoflagellate Amphidinium klebsii. Tetrahedron Lett 1995; 36: 6279–82.
Satake M, Murata M, Yasumoto T, Fujita T, Naoki H . Amphidinol, a polyhydroxy-polyene antifungal agent with an unprecedented structure, from a marine dinoflagellate, Amphidinium klebsii. J Am Chem Soc 1991; 113: 9859–61.
Irie T, Suzuki M, Masamune T . Laurencin, a constituent from laurencia species. Tetrahedron Lett 1965; 6: 1091–9.
Mao SC, Guo YW . A laurane sesquiterpene and rearranged derivatives from the Chinese red alga Laurencia okamurai Yamada. J Nat Prod 2006; 69: 1209–11.
Mao SC, Guo YW . Sesquiterpenes from algae of the Genus Larencia: chemistry and biological activities. Chin Tradit Herb Drugs 2004; 35: 8–18.
Mao SC, Guo YW, Shen X . Two novel aromatic valerenane-type sesquiterpenes from the Chinese green alga Caulerpa taxifolia. Bioorg Med Chem Lett 2006; 16: 2947–50.
Wang BS, Liang SC, Zhang WY, Zan QJ . Mangrove Flora of the world. Acta Bot Sin 2003; 45: 644–53.
Li MY, Xiao Q, Pan JY, Wu J . Natural products from semi-mangrove flora: source, chemistry and bioactivities. Nat Prod Rep 2009; 26: 281–98.
Sun YQ, Guo YW . Gymnorrhizol, an unusual macrocyclic polydisulfide from the Chinese mangrove Bruguiera gymnorrhiza. Tetrahedron Lett 2004; 45: 5533–5.
Sun YQ, Guo YW . Crystal structure of 1,2,6,7,11,12-hexathia-cyclopentadecane-4,9,14-triol, C9H18O3S6 . Z Kristallogr NCS 2004; 219: 121–3.
Gong JX, Shen X, Yao LG, Jiang H, Krohn K, Guo YW . Total synthesis of gymnorrhizol, an unprecedented 15-membered macrocyclic polydisulfide from the Chinese mangrove Bruguiera gymnorrhiza. Org Lett 2007; 9: 1715–6.
Huang XY, Wang Q, Liu HL, Zhang Y, Xin GR, Shen X, et al. Diastereoisomeric macrocyclic polydisulfides from the mangrove Bruguiera gymnorrhiza. Phytochemistry 2009; 70: 2096–100.
Jiang CS, Muller WE, Schroder HC, Guo YW . Disulfide- and multisulfide-containing metabolites from marine organisms. Chem Rev 2012; 112: 2179–207.
Cai YS, Kurtan T, Miao ZH, Mandi A, Komaromi I, Liu HL, et al. Palmarumycins BG1-BG7 and preussomerin BG1: establishment of their absolute configurations using theoretical calculations of electronic circular dichroism spectra. J Org Chem 2011; 76: 1821–30.
Wang L, Zhang CF, Wang ZT, Zhang M, Xu LS . Five new compounds from Dendrobium crystallinum. J Asian Nat Prod Res 2009; 11: 903–11.
Prajoubklang A, Sirithunyalug B, Charoenchai P, Suvannakad R, Sriubolmas N, Piyamongkol S, et al. Bioactive deoxypreussomerins and dimeric naphthoquinones from Diospyros ehretioides fruits: deoxypreussomerins may not be plant metabolites but may be from fungal epiphytes or endophytes. Chem Biodivers 2005; 2: 1358–67.
Ravindranath N, Ravinder Reddy M, Mahender G, Ramu R, Ravi Kumar K, Das B . Deoxypreussomerins from Jatropha curcas: are they also plant metabolites? Phytochemistry 2004; 65: 2387–90.
Kouam TNM, Lavaud C, Massiot G, Nuzillard JM, Connolly JD, Ryeroft DS . Bipendensin, an unusual phenolic acetal from Afzelia bipendensis. Nat Prod Lett 1993; 3: 299–303.
Cai YS, Guo YW, Krohn K . Structure, bioactivities, biosynthetic relationships and chemical synthesis of the spirodioxynaphthalenes. Nat Prod Rep 2010; 27: 1840–70.
Chen XL, Liu HL, Li J, Xin GR, Guo YW . Paracaseolide A, first alpha-alkylbutenolide dimer with an unusual tetraquinane oxa-cage bislactone skeleton from Chinese mangrove Sonneratia paracaseolaris. Org Lett 2011; 13: 5032–5.
Hill RA, Sutherland A . Hot off the press. Nat Prod Rep 2011; 28: 1879–82.
Liu HL, Huang XY, Li J, Xin GR, Guo YW . Absolute configurations of integracins A, B, and 15′-dehydroxy-integracin B. Chirality 2012; 24: 459–62.
Wang JD, Zhang W, Li ZY, Xiang WS, Guo YW, Krohn K . Elucidation of excogallochaols A–D, four unusual diterpenoids from the Chinese mangrove Excoecaria agallocha. Phytochemistry 2007; 68: 2426–31.
Qin S, Liang JY, Gu YC, Guo YW . Suffruticosine, a novel octacyclic alkaloid with an unprecedented skeleton from Securinega suffruticosa (Pall) Rehd. Tetrahedron Lett 2008; 49: 7066–9.
Wang JR, Liu HL, Kurtan T, Mandi A, Antus S, Li J, et al. Protolimonoids and norlimonoids from the stem bark of Toona ciliata var pubescens. Org Biomol Chem 2011; 9: 7685–96.
Jiang CS, Zhou R, Gong JX, Chen LL, Kurtan T, Shen X, et al. Synthesis, modification, and evaluation of (R)-de-O-methyllasiodiplodin and analogs as nonsteroidal antagonists of mineralocorticoid receptor. Bioorg Med Chem Lett 2011; 21: 1171–5.
Huang XC, Li J, Li ZY, Shi L, Guo YW . Sesquiterpenes from the Hainan Sponge Dysidea septosa. J Nat Prod 2008; 71: 1399–403.
Li Y, Zhang Y, Shen X, Guo YW . A novel sesquiterpene quinone from Hainan sponge Dysidea villosa. Bioorg Med Chem Lett 2009; 19: 390–2.
Zhang Y, Li Y, Guo YW, Jiang HL, Shen X . A sesquiterpene quinone, dysidine, from the sponge Dysidea villosa, activates the insulin pathway through inhibition of PTPases. Acta Pharmacol Sin 2009; 30: 333–45.
Mao SC, Manzo E, Guo YW, Gavagnin M, Mollo E, Ciavatta ML, et al. New diastereomeric bis-sesquiterpenes from Hainan marine sponges Axinyssa variabilis and Lipastrotethya ana. Tetrahedron 2007; 63: 11108–13.
Li L, Sheng L, Wang CY, Zhou YB, Huang H, Li XB, et al. Diterpenes from the Hainan soft coral Lobophytum cristatum Tixier-Durivault. J Nat Prod 2011; 74: 2089–94.
Zhang CX, Yan SJ, Zhang GW, Lu WG, Su JY, Zeng LM, et al. Cytotoxic diterpenoids from the soft coral Sinularia microclavata. J Nat Prod 2005; 68: 1087–9.
Li Y, Carbone M, Vitale RM, Amodeo P, Castelluccio F, Sicilia G, et al. Rare casbane diterpenoids from the Hainan soft coral Sinularia depressa. J Nat Prod 2010; 73: 133–8.
Jia R, Guo YW, Chen P, Yang YM, Mollo E, Gavagnin M, et al. Biscembranoids and their probable biogenetic precursor from the Hainan soft coral Sarcophyton tortuosum. J Nat Prod 2007; 70: 1158–66.
Yan XH, Gavagnin M, Cimino G, Guo YW . Two new biscembranes with unprecedented carbon skeleton and their probable biogenetic precursor from the Hainan soft coral Sarcophyton latum. Tetrahedron Lett 2007; 48: 5313–6.
Yan XH, Lin LP, Ding J, Guo YW . Methyl spongoate, a cytotoxic steroid from the Sanya soft coral Spongodes sp. Bioorg Med Chem Lett 2007; 17: 2661–3.
Gong JX, Miao ZH, Yao LG, Ding J, Kurtan T, Guo YW . Stereoselective synthesis of methyl spongoate, a new steroid with potent antitumor activities. Synlett 2010; 2010: 480–2.
Jiang CS, Huang CG, Feng B, Li J, Gong JX, Kurtan T, et al. Synthesis and antitumor evaluation of methyl spongoate analogs. Steroids 2010; 75: 1153–63.
Ciavatta ML, Manzo E, Mollo E, Mattia CA, Tedesco C, Irace C, et al. Tritoniopsins A–D, cladiellane-based diterpenes from the South China Sea nudibranch Tritoniopsis elegans and its prey Cladiella krempfi. J Nat Prod 2011; 74: 1902–7.
Gavagnin M, Mollo E, Docimo T, Guo YW, Cimino G . Scalarane metabolites of the nudibranch Glossodoris rufomarginata and its dietary sponge from the South China Sea. J Nat Prod 2004; 67: 2104–7.
Fontana A, Cavaliere P, Ungur N, D'Souza L, Parameswaram PS, Cimino G . New scalaranes from the nudibranch Glossodoris atromarginata and its sponge prey. J Nat Prod 1999; 62: 1367–70.
Terem B, Scheuer PJ . Scalaradial derivatives from the nudibranch chromodoris youngbleuthi and the sponge spongia oceania. Tetrahedron 1986; 42: 4409–12.
Manzo E, Gavagnin M, Bifulco G, Cimino P, Di MS, Ciavatta ML, et al. Aplysiols A and B, squalene-derived polyethers from the mantle of the sea hare Aplysia dactylomela. Tetrahedron 2007; 63: 9970–8.
Carbone M, Gavagnin M, Mattia CA, Lotti C, Castelluccio F, Pagano B, et al. Structure of onchidione, a bis-γ-pyrone polypropionate from a marine pulmonate mollusk. Tetrahedron 2009; 65: 4404–9.
Wang JR, Carbone M, Gavagnin M, Mandi A, Antus S, Yao LG, et al. Assignment of absolute configuration of bis-γ-pyrone polypropionates from marine pulmonate molluscs. Eur J Org Chem 2012; 2012: 1107–11.
Carbone M, Li Y, Irace C, Mollo E, Castelluccio F, Di Pascale A, et al. Structure and cytotoxicity of phidianidines A and B: first finding of 1,2,4-oxadiazole system in a marine natural product. Org Lett 2011; 13: 2516–9.
Manzo E, Carbone M, Mollo E, Irace C, Di Pascale A, Li Y, et al. Structure and synthesis of a unique isonitrile lipid isolated from the marine mollusk Actinocyclus papillatus. Org Lett 2011; 13: 1897–9.
Acknowledgements
The authors wish to acknowledge all of their colleagues who have contributed to the studies presented herein and to thank Prof T Kurtán (University of Debrecen, Hungary) for his contribution to the establishment of absolute stereochemistry by CD methodology. The reported research was financially supported by the National Marine '863' Project (No 2011AA09070102), the National Natural Science Foundation of China (No 21072204, 21021063, 40976048, 31070310, and 81072572), the SKLDR/SIMM Projects (No SIMM 1203ZZ-03 and SIMM 1105KF-04), and Syngenta-SIMM-PhD Studentship Project, and partially funded by the National S&T Major Project (2011ZX09307-002-03) and the EU 7th Framework Programme-IRSES Project (2010-2014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, Zf., Guo, Yw. Bioactive natural products from Chinese marine flora and fauna. Acta Pharmacol Sin 33, 1159–1169 (2012). https://doi.org/10.1038/aps.2012.110
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2012.110
Keywords
This article is cited by
-
Chemistry and bioactivity of secondary metabolites from South China Sea marine fauna and flora: recent research advances and perspective
Acta Pharmacologica Sinica (2022)
-
α-Glucosidase inhibitors from a mangrove associated fungus, Zasmidium sp. strain EM5-10
BMC Chemistry (2019)
-
Novel and Neuroprotective Tetranortriterpenoids from Chinese Mangrove Xylocarpus granatum Koenig
Scientific Reports (2016)
-
Celebrating the 80th anniversary of the Shanghai Institute of Materia Medica, Chinese Academy of Sciences (SIMM)
Acta Pharmacologica Sinica (2012)