Abstract
Aim:
The cerebral state index (CSI) was recently introduced as an electroencephalographic monitor for measuring the depth of anesthesia. We compared the performance of CSI to the bispectral index (BIS) as electroencephalographic measures of sevoflurane effect using two combined sigmoidal Emax models.
Methods:
Twenty adult patients scheduled for laparotomy were studied. After induction of general anesthesia, sevoflurane concentrations were progressively increased and then decreased over 70 min. An analysis of the BIS and CSI with the sevoflurane effect-site concentration was conducted using two combined sigmoidal Emax models.
Results:
The BIS and CSI decreased over the initial concentration range of sevoflurane and then reached a plateau in most patients. A further increase in sevoflurane concentration produced a secondary plateau in the pharmacodynamic response. The CSI was more strongly correlated with effect-site sevoflurane concentration (R2=0.95±0.04) than the BIS was (R2=0.87±0.07) (P<0.05). The individual Emax and Ceff50 (effect-site concentration associated with 50% decrease from baseline to plateau) values for the upper and lower plateaus were significantly greater for BIS (12.7±7.3, 1.6±0.4, and 4.2±0.5, respectively) than for CSI (3.4±2.2, 1.2±0.4, and 3.8±0.5, respectively) (P<0.05). The remaining pharmacodynamic parameters for the BIS and CSI were similar.
Conclusion:
The overall performance of the BIS and CSI during sevoflurane anesthesia was similar despite major differences in their algorithms. However, the CSI was more consistent and more sensitive to changes in sevoflurane concentration, whereas the measured BIS seemed to respond faster. The newly developed combined Emax model adequately described the clinical data, including the pharmacodynamic plateau.
Similar content being viewed by others
Introduction
The electroencephalogram (EEG) is commonly used to measure an anesthetic drug's effect on the central nervous system. Several processed EEG monitors have been developed to extract and process the information because it is challenging to analyze the real-time, raw electroencephalographic signal during anesthesia. The most extensively validated device used for this purpose is the bispectral index (BIS) monitor (Aspect Medical System, Newton, MA, USA). The cerebral state index (CSI), which is obtained by a cerebral state monitor (CSMTM Danmeter, Odense, Denmark), was recently introduced as a measure of the hypnotic component of anesthesia, and its usefulness has been previously evaluated1, 2, 3, 4, 5, 6, 7. However, there are no reports regarding the dose-response relationship between sevoflurane concentration and its effect in adults measured with CSI.
The administration of inhalational anesthetic drugs produces dose-response changes on EEG devices. When increasing or decreasing the anesthetic depth level, delays between the end-tidal concentration and the effect-site concentration occur. Pharmacokinetic-pharmacodynamic modeling can quantify these delays by incorporating the plasma-effect site equilibration rate constant (Ke0)8. A classical sigmoidal Emax model has been commonly used to describe the dose-response relationship between anesthetic drugs and electroencephalographic data because of the advantage of its high flexibility. However, as anesthetic doses increase, two pronounced pharmacodynamic plateaus appear in the dose-response curves of the processed EEG devices9, 10, 11. These biphasic dose-response curves suggest that the classical sigmoidal Emax model cannot reflect these data, and thus a new pharmacodynamic model needs to be developed.
The aim of this study is to quantify and compare the pharmacodynamic profile of sevoflurane's effect measured with the CSI and the BIS using a new model that combines two sigmoidal Emax models.
Materials and methods
Patients and anesthesia
After obtaining approval of the Institutional Review Board, informed written consent was obtained from all patients. Twenty patients (8 men and 12 women), American Society of Anesthesiologists physical status I, ages 30 to 65 years old, were prospectively studied. All patients were scheduled for open subtotal gastrectomy under general anesthesia and did not receive any premedication. The patients' exclusion criteria were preoperative use of medication acting on the central nervous system or of opioids, excessive weight, a history of cardiac, pulmonary, hepatic, renal, or cerebrovascular disease, and use of any premedication. All subjects were fasted for at least 8 h before anesthesia.
After arrival at the operating room, standard monitors (ie, electrocardiogram, oxygen saturation, intermittent non-invasive blood pressure, and gas analysis) were applied. The EEG was continuously recorded using the BIS and the CSI monitor simultaneously for all patients. For the BIS electrodes, the skin of the forehead was prepared with alcohol swabbing, and monitoring electrodes were positioned as recommended by the manufacturer. We placed the BIS sensor with electrode 1 at the center of the forehead, approximately 5 cm above the nose, electrode 2 lateral to electrode 1, electrode 3 on temple between the corner of the eye and hairline, and electrode 4 right above the eyebrow. For the CSI electrodes, the skin of the forehead and mastoid process were firmly rubbed with abrasive paper, and one drop of sodium chloride was applied to the skin before placing the electrodes. One CSI electrode was placed at the midline of the forehead, one more laterally on the forehead, and one on the mastoid process behind the ear. After an initial control for electrode impedance, the monitor calculated its index from the raw EEG signals. Both monitors provided numerical indices from 0 to 100. Anesthesia was induced with 1.5 mg/kg propofol with a remifentanil infusion at 0.2 μg/kg per min. Rocuronium 0.6 mg/kg was administered to facilitate orotracheal intubation. Anesthesia was maintained with sevoflurane and remifentanil infusion at 0.2 μg/kg per min. Complete neuromuscular block was achieved by repeated injection of 0.2 mg/kg rocuronium and monitored by using neuromuscular monitoring, ie, train-of-four. The tidal volume was set at 8 mL/kg, and the respiratory rate was adjusted to maintain an end-tidal carbon dioxide partial pressure of 35–40 mmHg. The oral temperature was monitored continuously and was maintained at normothermia (36.0–36.5 °C) by using a warm blanket.
Study measurements
To rule out a residual propofol effect and to ensure a condition of constant surgical stimulation, the study measurements were performed during the opening of the peritoneum, a minimum of 45 min after the induction of anesthesia. The sevoflurane agent was administered using a sevoflurane vaporizer, and the sevoflurane was added to the inspired gas mixture. The end-tidal sevoflurane concentrations (ie, expired sevoflurane concentrations) were continuously measured with a Capnomac anesthetic gas analyzer (Datex, Copenhagen, Denmark) by sampling gas from the circuit. Sampling was performed as close to the patient as possible to minimize the effects of circuit dead space. It was assumed that the end-tidal sevoflurane concentration reflected its plasma concentration. The fresh gas flow was set at 6 L/min (4 L/min air and 2 L/min O2). To obtain concentration-response curves, the sevoflurane end-tidal concentrations were steadily increased and then decreased according to the following paradigm: starting at an end-tidal sevoflurane concentration of 1%, the sevoflurane vaporizer was increased stepwise by 0.5% every 2.5 min up to a maximum value of 8.0%. Subsequently, the sevoflurane vapor setting was decreased stepwise by 0.5% every 2.5 min again, until the end-tidal sevoflurane concentration had decreased to 1.0%, or a BIS value of 60 had been reached. The BIS and CSI values were recorded simultaneously during the study period as the sevoflurane end-tidal concentration changed. If necessary, phenylephrine was intermittently given for hemodynamic stability. After the final suture was placed, sevoflurane was discontinued, and the patients were allowed to awaken from anesthesia. All patients were examined for unintended wakefulness by an interview on postoperative d 1.
Pharmacodynamic model
Using a program called NONMEM VI, we modeled a relationship between the end-tidal concentrations of sevoflurane as an independent parameter and the BIS and CSI as dependent parameters. The model parameters were estimated by maximizing the likelihood between the measured and the predicted EEG parameters. To eliminate hysteresis between the end-tidal concentrations of sevoflurane and BIS and CSI values, an effect-site compartment was introduced into the model. Sevoflurane effect-site concentrations were obtained by simultaneous pharmacokinetic and pharmacodynamic modeling8. The effect site was assumed to be linked to the plasma compartment by a traditional first-order process, and the effect-site concentrations over time were calculated as the convolutions of the predicted plasma concentrations over time, with the disposition function of the effect site. The convolution was based on a “connect the dots” approach, previously used by Schnider et al12. The sevoflurane effect-site concentration was estimated using a differential equation as follows:

where Cet was the end-tidal concentration, Ceff was the effect-site concentration of sevoflurane, and ke0 was the first-order rate constant determining the equilibration between the two. The ke0 was estimated by minimizing the area of the hysteresis loop of either EEG index's data versus the effect-site concentration. One individual ke0 value was calculated for each patient on the basis of his or her particular inhalation anesthetic ramp.
Because of the bimodal shape of the concentration versus effect curves for all study subjects, the relationship between Ceff and the electroencephalographic effect E (BIS or CSI) was modeled using two combined sigmoid Emax models (E1 and E2):

The combined sigmoidal curve was made up of total sum of two separate Emax curves, indicated by E1 and E2. Both sigmoidal curves had their own parameters for E1 and E2. The effect (E) was the measured BIS and CSI value, E01 and E02 were the baseline values, Emax1 and Emax2 were the maximum values, λ1 and λ2 were the steepnesses of the concentration-response relation curves for E1 and E2, respectively, and Ceff was the calculated effect-site concentration of sevoflurane. Ceff501 and Ceff502 were the effect-site concentrations associated with a 50% decrease from E01 and E02 to Emax1 and Emax2, respectively. E1 was expected to represent the EEG effect of sevoflurane concentrations from the baseline value to the first plateau value, as E2 was constant in that period. E2 was expected to represent the EEG effect of sevoflurane from the first plateau value to the maximum value when E1 was constant.
The interindividual variability for E1 and E2 was assumed to have a log-normal distribution. The interindividual variabilities in E01, E02, Emax2, Ceff501, Ceff502, and ke0 were modeled using an exponential error model. Residual intraindividual variability was modeled using an additive error model. The FOCE with INTERACTION estimation method was used for analysis.
Covariate analysis
In our modeling approach, the base population model was first developed, which did not include any covariates. The effects of age, weight, height, and sex were then explored. The Bayesian estimates of the individual pharmacodynamic parameters were plotted against the covariates. Covariates were added one at a time and were kept in the model if they improved the goodness of the fit, judged by a reduction in the log likelihood ratio greater than 4 for each added degree of freedom, with P<0.05.
Statistical analysis
Minimizing the squared error between the measured and the predicted concentration necessarily maximizes the coefficient of determination (R2), which can be considered as an objection function:

The sum of squared errors (SSE) represents the sum of squares of the differences between the observed measurement, yi, for a given time and the corresponding model prediction, ŷi. The total sum of squares (SST) indicates the sum of squares of the differences between each actual measurement, yi, and the average of all measurements, ÿi. The R2 values and pharmacodynamic parameters were compared between the two indices using Student's t test or the Wilcoxon test where appropriate. All tests were two-tailed, with statistical significance defined as P<0.05. Data are presented as mean and standard deviation (SD). Statistical analysis was performed using Sigma Stat 2.03 and Sigma Plot 2000 (SPSS Inc, Erkrath, Germany) and SPSS (version 12) computer software.
Results
Twenty patients, 8 men and 12 women, age 52±9 years old, weight 60±8 kg, and height 166±8 cm, were enrolled in this study. There were no problems with the skin adherence of the electrodes. The systolic blood pressure was maintained above 90 mmHg during the study period in all patients. No patients reported postoperative recall of intraoperative awareness. The mean duration of the measurements was 59.2±8.4 min. The end-tidal sevoflurane concentration ranged from 0.96%±0.15% to 5.30%±0.46%.
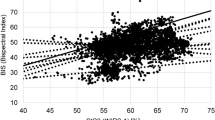
Figure 1 shows the observed value and predicted value of the CSI vs time (A) and the effect-site sevoflurane concentration (B) from one patient. Figure 2 shows the time course of the measured BIS (A) and CSI (B) obtained from all patients in the study. The hysteresis loop between the BIS, CSI, and end-tidal sevoflurane concentration is illustrated in Figure 3. Decreased BIS and CSI values adequately reflected the increase in the sevoflurane effect-site concentrations, and sevoflurane revealed a pronounced plateau, leading to a biphasic dose-response curve in most cases of the BIS and CSI (Figure 4A and 4C). In one patient who was included in our results, an unexpectedly prolonged paradoxical BIS elevation was observed when the effect-site concentration of sevoflurane was high (>3.5%), but the CSI values were similar to those of the population group. The observed values and individually predicted values of the BIS and CSI against the calculated effect-site sevoflurane concentration are shown, and a combined sigmoidal Emax model was fitted to the data from all patients (Figure 4). The goodness of the model's fit was analyzed by the relationship between the observed values and the individually predicted values, and a smaller scatter was observed for the CSI than for the BIS (Figure 5). The correlation of the CSI to the effect-site sevoflurane concentration (R2=0.95±0.04) was significantly better than that of the BIS to the effect-site sevoflurane concentration (R2=0.87±0.07) (P<0.05).
Observed value and predicted value of CSI vs time (A) and effect-site sevoflurane concentration (B) from one patient. The individual prediction value (E) is the sum of the two sigmoidal Emax models (E1 and E2). E1 represents the effect of sevoflurane concentrations from the baseline value to plateau value, while E2 remains constant. E2 represents the effect at sevoflurane concentrations from the plateau value to the maximal value while E1 remains constant.
The pharmacodynamic parameters are displayed in Table 1. The Emax1 value was fixed at 0 in our study because E02 can represent a part of a pharmacodynamic plateau, and a more stable model can be produced without overparametrization. The Ke0 value of the individual fits derived from the BIS was 0.36±0.48 min−1, and that from the CSI was 0.22±0.16 min−1(P>0.05). Both the Ceff501 and Ceff502 values of the BIS were greater than those of the CSI (P<0.05). The individual Emax2 value for the CSI (3.4±2.2) was significantly lower than that for the BIS (12.7±7.3)(P<0.05). The intraindividual variabilities (residual error σ) for the BIS and CSI were 5.7 and 4.3, respectively. During the covariate model selection, none were found to be significant for improving the model's fit to the data.
Discussion
This study demonstrated that the CSI has only minor differences in dose-response relations, compared with the BIS, as a measure of the electroencephalographic effects of sevoflurane, despite major differences in their algorithms. The overall BIS and CSI progressively decreased with the increasing sevoflurane concentration, but the prominent pharmacodynamic plateau was observed in most cases, necessitating the use of this new Emax model. The newly developed combined sigmoidal Emax model adequately described the pharmacodynamic parameters in the processed EEG data, including the pharmacodynamic plateau.
The CSI uses an algorithm different from that used for the BIS, which has four subparameters derived from the time domain analysis (burst-ratio) and frequency domain analysis (α-ratio, β-ratio, and β-ratio-α-ratio) of the EEG. Some comparative studies between the BIS and CSI have shown similar values with both monitors during administration of propofol and inhalational agents1, 2, 3, 5, 6, 7. However, our study is the first clinical investigation of pharmacodynamic modeling using the CSI for adult patients. Pharmacokinetic-pharmacodynamic modeling is required before using new EEG devices as tools to guide anesthetic administration and to quantify the drug concentration versus the effect relationship13. This approach can quantify the relationship between the effect-site concentration and measured effect with these monitors, as well as the speed of onset and offset of the anesthetic's action.
When using a processed parameter from the EEG, the larger the ke0 estimated value is, the faster the on and offset times for the response measured with the monitor are. The ke0 in this study represents not only sevoflurane's end-tidal to brain equilibration time, but also the time delay of the index used to measure the sevoflurane effect2. The ke0 was faster with the BIS than with the CSI, although the two values were not statistically different. Our results were similar to those reported in children whose Ke0 value in the BIS was also greater than in the CSI, although the actual Ke0 values of children are larger than those of adults2, 14. Our mean individual Ke0 value for the BIS was 0.36 min−1, which is comparable to previously reported range (0.22–0.48 min−1)15, 16, 17, 18. While it was expected that the administration of additional remifentanil might lead to an increase in ke017, the infusion dose of remifentanil did not seem to have much influence on the ke0 value. The BIS has higher interindividual variability in the coefficients of variation of the Emax2 than the CSI. This finding suggests that the BIS may be a less consistent indicator for deep levels of anesthetic than the CSI. Both Ceff501 and Ceff502 values of the BIS are greater than those of the CSI, meaning that the CSI shows higher sensitivity to changes in sevoflurane concentrations in the periods before and after the pharmacodynamic plateau.
Patient covariates may have some influence on the dynamic profile of sevoflurane's effect, although this influence has not been previously investigated. The Ceff50 and ke0 were expected to decrease with increasing age because age reduces the requirements of sevoflurane and increases the blood-brain equilibration times14. However, in our population, the covariate of age did not improve the goodness of fit. Thus, a study population with a broader age distribution would be required to clarify whether the pharmacodynamic parameters are dependent on age.
We chose the R2 value as an indicator of the relationship between the EEG signal and sevoflurane concentration. The R2 value of the CSI, with values approaching 1, suggests that the changes in electroencephalographic effect can be entirely explained by changes in anesthetic concentration at its postulated effect site. The R2 value of the BIS of 0.87 is likely influenced by an unexpected case, which greatly deviated from the mean population group but was not excluded.
The pharmacodynamic plateau appears at the time of transition to burst suppression on the BIS monitor19. At the first attempt, the minimization of NONMEM was not successful for either the BIS data or CSI data using the classical sigmoidal Emax model, which could not adequately quantify the dose-response relationship (data not shown). Some studies have explained the EEG data showing a pharmacodynamic plateau with Emax models that are divided into two periods, before and after the onset of burst suppression9, 10. The pharmacodynamic plateau appeared when the BIS value was approximately 40 during sevoflurane anesthesia10. Our model may represent the pharmacodynamic plateau with the E02 value, as it was designed with the Emax1 value fixed at 0. The mean individual predictive E02 value of 38.7 for the BIS, close to 40 as shown in other studies, suggests that the transition point into burst suppression can be properly described with our model. The pharmacodynamic plateau in the BIS may be more prominent with anesthetics when the duration of one sequence for increasing or decreasing concentrations of the anesthetic agent is short, which is more clinically applicable9, 10, 11.
Limitations of our study include that the λ1 and Ceff501 of the BIS and CSI are likely to be different from data collected on induction or data including low sevoflurane concentrations, as our experiment precluded the use of less than 1% sevoflurane end-tidal concentrations. The Ceff501 and λ1 may correspond to the Ceff50 and λ in data that exclude the burst suppression or deep anesthetic condition. The Ceff501 and λ1 of the BIS in our study were somewhat greater than the Ceff50 and λ in previously published research16, 18. However, our results may be more common from a clinical point of view.
In conclusion, the overall performance of both monitors during sevoflurane anesthesia was similar, despite major differences in their algorithms. However, the CSI was more consistent and more sensitive to changes in sevoflurane concentration, whereas the measured BIS seemed to respond more quickly. The newly developed combined sigmoidal Emax model adequately described the pharmacodynamic parameters in the processed EEG data, including the pharmacodynamic plateau. This model could be further investigated for pharmacodynamic data, including the burst suppression plateau.
Author contribution
Dong Woo HAN and Jong Seok LEE designed research; Jun Ho KIM performed research; Dong Woo HAN, Olinto-Jose LINARES-PERDOMO, and Steven E KERN analyzed data; and Dong Woo HAN wrote the paper.
References
Anderson RE, Jakobsson JG . Cerebral state monitor, a new small handheld EEG monitor for determining depth of anaesthesia: a clinical comparison with the bispectral index during day-surgery. Eur J Anaesthesiol 2006; 23: 208–12.
Fuentes R, Cortínez LI, Struys MM, Delfino A, Muñoz H . The dynamic relationship between end-tidal sevoflurane concentrations, bispectral index, and cerebral state index in children. Anesth Analg 2008; 107: 1573–8.
Hoymork SC, Hval K, Jensen EW, Raeder J . Can the cerebral state monitor replace the bispectral index in monitoring hypnotic effect during propofol/remifentanil anaesthesia? Acta Anaesthesiol Scand 2007; 51: 210–6.
Anderson RE, Jakobsson JG . Cerebral state index response to incision: a clinical study in day-surgical patients. Acta Anaesthesiol Scand 2006; 50: 749–53.
Cortínez LI, Delfino AE, Fuentes R, Muñoz HR . Performance of the cerebral state index during increasing levels of propofol anesthesia: a comparison with the bispectral index. Anesth Analg 2007; 104: 605–10.
Jensen EW, Litvan H, Revuelta M, Rodriguez BE, Caminal P, Martinez P, et al. Cerebral state index during propofol anesthesia: a comparison with the bispectral index and the A-line ARX index. Anesthesiology 2006; 105: 28–36.
Zhong T, Guo QL, Pang YD, Peng LF, Li CL . Comparative evaluation of the cerebral state index and the bispectral index during target-controlled infusion of propofol. Br J Anaesth 2005; 95: 798–802.
Sheiner LB, Stanski DR, Vozeh S, Miller RD, Ham J . Simultaneous modeling of pharmacokinetics and pharmacodynamics: application to d-tubocurarine. Clin Pharmacol Ther 1979; 25: 358–71.
Kreuer S, Bruhn J, Larsen R, Grundmann U, Shafer SL, Wilhelm W . Application of bispectral index and narcotrend index to the measurement of the electroencephalographic effects of isoflurane with and without burst suppression. Anesthesiology 2004; 101: 847–54.
Kreuer S, Bruhn J, Walter E, Larsen R, Apfel CC, Grundmann U, et al. Comparative pharmacodynamic modeling using bispectral and narcotrend-index with and without a pharmacodynamic plateau during sevoflurane anesthesia. Anesth Analg 2008; 106: 1171–81.
Kreuer S, Bruhn J, Ellerkmann R, Ziegeler S, Kubulus D, Wilhelm W . Failure of two commercial indexes and spectral parameters to reflect the pharmacodynamic effect of desflurane on EEG. J Clin Monit Comput 2008; 22: 149–58.
Schnider TW, Minto CF, Shafer SL, Gambus PL, Andresen C, Goodale DB, et al. The influence of age on propofol pharmacodynamics. Anesthesiology 1999; 90: 1502–16.
Billard V, Gambus PL, Chamoun N, Stanski DR, Shafer SL . A comparison of spectral edge, delta power, and bispectral index as EEG measures of alfentanil, propofol, and midazolam drug effect. Clin Pharmacol Ther 1997; 61: 45–58.
Cortínez LI, Trocóniz IF, Fuentes R, Gambús P, Hsu YW, Altermatt F, et al. The influence of age on the dynamic relationship between end-tidal sevoflurane concentrations and bispectral index. Anesth Analg 2008; 107: 1566–72.
Ellerkmann RK, Liermann VM, Alves TM, Wenningmann I, Kreuer S, Wilhelm W, et al. Spectral entropy and bispectral index as measures of the electroencephalographic effects of sevoflurane. Anesthesiology 2004; 101: 1275–82.
Soehle M, Ellerkmann RK, Grube M, Kuech M, Wirz S, Hoeft A, et al. Comparison between bispectral index and patient state index as measures of the electroencephalographic effects of sevoflurane. Anesthesiology 2008; 109: 799–805.
Olofsen E, Sleigh JW, Dahan A . The influence of remifentanil on the dynamic relationship between sevoflurane and surrogate anesthetic effect measures derived from the EEG. Anesthesiology 2002; 96: 555–64.
Mourisse J, Lerou J, Struys M, Zwarts M, Booij L . Multi-level approach to anaesthetic effects produced by sevoflurane or propofol in humans: 1. BIS and blink reflex. Br J Anaesth 2007; 98: 737–45.
Bruhn J, Bouillon TW, Shafer SL . Onset of propofol-induced burst suppression may be correctly detected as deepening of anaesthesia by approximate entropy but not by bispectral index. Br J Anaesth 2001; 87: 505–7.
Acknowledgements
This work was supported in part by the 2007 Yonsei University Research Fund. The authors thank Tae Dong KWEON, MD (Department of Anesthesiology and Pain Medicine and Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Seoul, Korea), for his assistance with the data collection and design of the research protocol. This work was performed at Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, D., Linares-Perdomo, OJ., Lee, J. et al. Comparison between cerebral state index and bispectral index as measures of electroencephalographic effects of sevoflurane using combined sigmoidal Emax model. Acta Pharmacol Sin 32, 1208–1214 (2011). https://doi.org/10.1038/aps.2011.99
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2011.99
Keywords
This article is cited by
-
Evaluating NeuroSENSE for assessing depth of hypnosis during desflurane anesthesia: an adaptive, randomized-controlled trial
Canadian Journal of Anesthesia/Journal canadien d'anesthésie (2020)