Abstract
Aim:
To explore the alteration of endogenous metabolites and identify potential biomarkers using metabolomic profiling with gas chromatography coupled a time-of-flight mass analyzer (GC/TOF-MS) in a rat model of estrogen-deficiency-induced obesity.
Methods:
Twelve female Sprague-Dawley rats six month of age were either sham-operated or ovariectomized (OVX). Rat blood was collected, and serum was analyzed for biomarkers using standard colorimetric methods with commercial assay kits and a metabolomic approach with GC/TOF-MS. The data were analyzed using multivariate statistical techniques.
Results:
A high body weight and body mass index inversely correlated with serum estradiol (E2) in the OVX rats compared to the sham rats. Estrogen deficiency also significantly increased serum total cholesterol, triglycerides, and low-density lipoprotein cholesterol. Utilizing GC/TOF-MS-based metabolomic analysis and the partial least-squares discriminant analysis, the OVX samples were discriminated from the shams. Elevated levels of cholesterol, glycerol, glucose, arachidonic acid, glutamic acid, glycine, and cystine and reduced alanine levels were observed. Serum glucose metabolism, energy metabolism, lipid metabolism, and amino acid metabolism were involved in estrogen-deficiency-induced obesity in OVX rats.
Conclusion:
The series of potential biomarkers identified in the present study provided fingerprints of rat metabolomic changes during obesity and an overview of multiple metabolic pathways during the progression of obesity involving glucose metabolism, lipid metabolism, and amino acid metabolism.
Similar content being viewed by others
Introduction
Obesity is a medical condition in which excess body fat has accumulated to such an extent1 that it becomes linked to several common diseases, including insulin resistance2, cardiovascular disease3, stroke4, and certain cancers5, 6, 7, 8. Apart from the genetic, environmental, and nutritional factors involved in the occurrence of obesity, endogenous estrogens play an important role in the regulation of body weight and obesity9, 10. The decrease in estrogen levels experienced by women after menopause could be associated with an increased risk of obesity and a series of devastating diseases11, 12. The relationship between obesity and endogenous estrogen remains unclear. Similar to leptin, estrogens regulate body weight and energy metabolism13. Moreover, ovariectomy-induced adiposity is associated with decreased energy expenditure, adipose tissue expansion, and hepatic steatosis14. Estrogen receptors alpha and beta play essential roles in controlling the energy metabolism pathways, body fat distribution, and normal body weight.
Estrogen replacement therapy (ERT), the medical administration of estrogen, prevents obesity and estrogen-deficiency-induced complications15, 16. In addition, estrogen has favorable effects on the lipid profile, significantly increasing high-density lipoprotein cholesterol (HDL-C) and reducing low-density lipoprotein cholesterol (LDL-C). ERT helps to relieve the cardiovascular diseases caused by obesity by decreasing cardiac mortality, improving endothelial function, and reducing LDL accumulation17, 18. However, the safety of ERT is controversial, although appropriate amounts of estrogen moderate estrogen-deficiency-induced obesity in postmenopausal women. The extensive use of estrogens results in a significant increase in the incidence of breast cancer19 and ovarian cancer20 in postmenopausal women. In addition, an increased risk of deep vein thrombosis (DVT)21 and dementia has been reported when estrogen alone is used in postmenopausal women aged 65 years or older based on data from the Women's Health Initiative (WHI)22. Therefore, an understanding of the comprehensive metabolic disorders and metabolic syndromes of estrogen-deficiency-induced obesity has become a topic of interest.
Metabonomics/metabolomics is defined as “the quantitative measurement of the dynamic multiparametric responses of a living system to pathophysiological stimuli or genetic modification”23. It is a novel “-omics” discipline that monitors alterations in the levels of endogenous metabolites in response to biological stimuli using modern spectroscopic techniques and statistical approaches24. Compared with genomics, transcriptomics, and proteomics, metabolomics profiling can provide a holistic overview and time-dependent snapshots of the physiologic process of endogenous metabolites in cells, tissues, organs, or organisms25. As such, metabolomics has been comprehensively applied in physiology, disease, and toxicology research fields26. Nuclear magnetic resonance (NMR) spectroscopy27, liquid chromatography coupled mass spectrometry (LC-MS)28, gas chromatography coupled mass spectrometry (GC-MS)29, and Fourier transform mass spectrometry30 have been successfully used to obtain metabolomic data. GC coupled to a time-of-flight mass analyzer (GC-TOF/MS) provides an improvement over conventional GC-MS analysis due to its high scan rates and the availability of mathematic algorithms to deconvolute closely overlapping peaks. Recent metabolomic approaches utilizing GC-TOF/MS for high-throughput analysis have been successfully applied to the study of hypertension31, hyperlipemia32, colon carcinoma, and professional athletes33 in serum or urine from rat and humans34. Therefore, for accuracy and thoroughness, a GC/TOF-MS-based metabolomics approach was implemented to study the biochemical changes in the serum of estrogen-deficiency-induced obesity in female rats in our study. A bilateral ovariectomy was applied to induce estrogen deficiency in female rats. A GC/TOF-MS spectrogram of rat serum was analyzed using a multivariate statistical analysis (MVSA) to differentiate the stages of obesity in OVX rats. The alterations of endogenous metabolites were examined under the conditions of estrogen-deficiency-induced obesity.
Materials and methods
Chemicals and reagents
Myristic-1,2-13C2 acid, 99 atom %13C was used as an internal standard (IS). Methoxyamine hydrochloride (purity 98%), alkane standard solution (C8-C40), and pyridine (>99.8% GC grade) were purchased from Sigma-Aldrich Corporation (St Louis, MO, USA).
N-methyl-N-trimethylsilyl-trifluoroacetamide (MSTFA) plus 1% trimethyl chlorosilane (TMCS) were obtained from Fluka (Buchs, Switzerland). Methanol (Merck, Darmstadt, Germany) and n-heptane (Tedia, Fairfield, OH, USA) were of high pressure liquid chromatography (HPLC) grade.
Purified water was produced by a Milli-Q system (Millipore, Milford, MA, USA). Kits for total cholesterol (TC), triglyceride (TG), HDL-C and LDL-C were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China). An ELISA kit for estradiol (E2) was purchased from R&D Systems (Minneapolis, MN, USA).
Animals, surgery and sample preparation
Twelve virgin specific pathogen-free Sprague-Dawley female rats (6 months old, body weight 250±10.0 g) were obtained from the Animal Center of Nanjing Medical University. Rats were housed under controlled conditions (room temperature 22±1°C) with standard solid food and water provided ad libitum during the experiment. This study was reviewed and approved by the Animal Ethical Committee of China Pharmaceutical University.
Bilateral ovariectomy (OVX, n=6) or sham operations (sham, n=6) were performed after a seven-day acclimatization. A bilateral (left and right) incision (1–2 cm) that included the skin, muscle, and peritoneum was performed 2 cm below the last rib, and the left and right ovaries were extirpated. For the sham operation, only a small amount of fat around each ovary was extirpated. The rats were provided with standard solid food and deionized water following surgery. Body weights and body mass indexes36 (BMI, a measurement comparing weight and the height squared) of rats were measured weekly and blood samples were collected on day 0 prior to the operation and at the 3rd and 6th week after bilateral ovariectomy. Serum was immediately separated by centrifugation at 2000×g for 10 min at 4 °C after whole blood was placed for 20 min, and was aliquoted into two parts. One aliquot was used to assay the levels of serum E2 by ELISA and TC, TG, HDL-C, and LDL-C using standard colorimetric methods with commercial kits. The other aliquot was used for GC/TOF-MS analysis. All samples were stored at -80 °C until analysis. All rats were weighed, anesthetized, and bled from the carotid arteries at the end of the experiments 6 weeks post-surgery. Uterine tissues were removed and weighed, and uterus indexes were calculated by dividing the uterus weight by the body weight.
Sample preparation for GC-TOF/MS analysis
Two hundred microliters of methanol containing the internal standard myristic-1,2-13C2 acid (12.5 μg/mL) was added to deproteinize a 50-μL serum sample. After vortex mixing for 5 min and centrifugation at 20 000×g for 10 min at 4 °C, 100 μL of supernatant was injected directly into a GC vial and then evaporated to dryness under vacuum. Subsequently, 30 μL of methoxyamine in pyridine (15 mg/mL) was added to the residue and vortexed for 2 min. A methoximation reaction was performed at room temperature for 16 h before trimethylsilylation was performed by the addition of 30 μL MSTFA with 1% TMCS as catalyst for 1 h. Finally, the solution was vortexed for 30 s after methyl myristate in heptane (30 μg/mL), the external standard, was added to a GC vial for GC-TOF/MS analysis33, 34, 35.
GC-TOF/MS analysis
An Agilent 6980 GC was equipped with an Agilent 7683 Series autosampler (Agilent, Atlanta, GA). Chromatographic separation was performed on a fused-silica capillary column (10 m×0.18 mm ID) chemically bonded with a 0.18 μm DB5-MS stationary phase (J&W Scientific, Folsom, CA, USA). Mass spectra were obtained using a Pegasus III TOF/MS (Leco). The injector temperature and the constant flow rate of carrier gas were controlled at 250 °C at a rate of 1 mL/min with the following parameters: a gradient temperature programming of 70 °C (2.0 min), 70–310 °C linear (35 °C/min), 310 °C (2.0 min); a purge time and flow rate of 60 s at 20 mL/min for 1 min; transfer line temperature and ion source temperature set at 250 °C and 200 °C, respectively; ion source voltage and current of 70 eV and 3.0 mA. The mass spectrometric data were acquired in scan mode over a range of m/z 50–800 at a rate of 20 spectra/s, and the acceleration voltage was turned on at -1650 V with a solvent delay of 170 s.
Data analysis
Automatic peak detection and calculation of the peak area of specific compounds and IS were analyzed by ChromaTOF 2.00 software (Leco). Peaks with signal-to-noise (S/N) ratios lower than 30 were rejected35. The retention index for each peak or compound was obtained by comparing its retention time against those of the alkane series (C8-C40). Identification of all of the compounds was performed by comparing their mass spectra and retention indexes with the authentic reference standards and the standards listed in the NIST 2.0 (2005), the Wiley library, and the in-house mass spectra library database established in the Key Laboratory of Drug Metabolism and Pharmacokinetics at China Pharmaceutical University. In addition, potential metabolites were searched using the Human Metabolome Database (http://www.hmdb.ca).
Multivariate statistical analysis (MVSA) was performed using SIMCA-P 11 software (Umetrics, Umea°, Sweden). The data matrix was constructed by GC-TOF/MS responses of each peak as variables with the sample names/IDs as observations in columns and each of the peaks in rows. Subsequently, a principal component analysis (PCA) and a partial least-squares–discriminant analysis (PLS-DA) were used to process the acquired data from the GC-TOF/MS analysis33.
All data are expressed as means±SD, and statistical analyses were performed using Student's t-test for independent two-sample and one-way ANOVA for the comparison of multiple means. P<0.05 indicated statistical significance.
Results
Body weight, body mass, and uterine index
Body weight, BMI, and the uterine index were determined; the results are listed in Figure 1A–1C). A significant increase (P<0.05) in body weight from the third week post-operation was observed in the OVX group (Figure 1A). Body weight was significantly elevated (P<0.001) in the OVX group compared to the sham group in the 4th, 5th, and 6th weeks after surgery. In addition, BMI was significantly increased in the OVX group in the 3rd (P<0.05) and 6th (P<0.001) weeks compared to the sham group (Figure 1B). Week 6 post-operation, the level of the uterine index was significantly lower (P<0.001) than those in the sham group (Figure 1C).
Body weight, body mass index, and uterus index from OVX and the sham rats. (A) Body weight of the rats was recorded weekly during the experiment. (B) Body mass index observed in the pre-surgery and weeks 3 and 6 post-surgery groups. (C) Uterus index. Values with a superscript are significantly different from the sham group (bP<0.05, cP<0.01).
Serum biochemical analysis
As shown in Table 1, serum TC levels in the OVX group in weeks 3 and 6 increased significantly after surgery compared to the sham group (P<0.05 and P<0.01, respectively). Compared with pre-surgery levels, serum TC (P<0.001) and TG (P<0.05 and P<0.001, respectively) levels were significantly increased in weeks 3 and 6 post-bilateral OVX. Similar to TC and TG, serum LDL-C levels in the OVX group in week 6 post-operation were significantly elevated (P<0.001) compared to the sham group and the OVX group prior to surgery. In addition, HDL-C levels in the OVX group observed at weeks 3 and 6 post-surgery were slightly reduced but showed no statistical difference compared with the OVX group prior to surgery or with the sham group.
Metabolomic analysis of plasma samples in rats with estrogen-deficiency-induced obesity
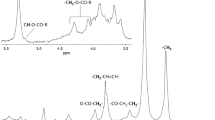
The total ion current chromatograms produced by GC-TOF/MS analysis are shown in Figure 2. More than 110 compounds, including amino acids, organic acids, saccharides, and fatty acids, were identified in the metabolomic profiling. In our experiment, the relative standard deviations of the internal standard and the external standard samples were 4.38% and 3.47%, respectively.
GC-TOF/MS chromatograph of a serum sample obtained from a sham-operated rat. 1, Alanine; 2, Lactic acid; 3, β-Hydroxybutyric acid; 4, Valine; 5, Leucine; 6, Glycerol; 7, Phosphoric acid; 8, Glycine; 9, Isoleucine; 10, Cysteine; 11, Glyceraldehyde 3-phosphate; 12, Creatinine; 13, Glutamic acid; 14, Glutamine; 15, Glucose; 16, Hexadecanoic acid; 17, Uric acid; 18, Oleic acid; 19, Octadecadienoic acid; 20, Octadecanoic acid; 21, Arachidonic acid; 22, Cholesterol.
PLS-DA was performed on the acquired metabolomic dataset. The PLS-DA score plot for the whole data set categorized serum samples into pre-surgery and weeks 3 and 6 post-surgery in the OVX group (Figure 3A). All of the serum samples in the metabolomic profile of the OVX group (pre-surgery and weeks 3 and 6) were calculated using PLS-DA. Subsequently, a PLS-DA model of two principal components could explain 59.8% of the GC-TOF/MS response variables and predict 72.5% and explain 95.3% according to R2X, R2Y, and Q2Y parameters (R2X is the percentage of all GC/TOF-MS response variables explained by the model. R2Y is the percentage of all observation or sample variables explained by the model. Q2Y is the percentage of all observation or sample variables predicted by the model33). The scores are plotted in Figure 3A, in which the x-axis and y-axis are labeled PC1 (the first principal component) and PC2 (the second principal component), respectively. Time-dependent changes of the scores plot in the serum metabolomic profile are obvious in the 2D plane graph. The scores plot shows the samples from rats prior to surgery clustered in the right area of the plot. However, the samples from rats 3 weeks post-surgery are gathered in the top left quadrant of the plot. The samples from rats in week 6 are in the bottom left quadrant of the plot. Our analysis indicated that the stages of estrogen-deficiency-induced obesity could be differentiated clearly between pre-surgery and weeks 3 and 6 post-surgery. Fold changes of endogenous metabolites were further studied by the loadings plot (not shown); the results are shown in Table 2. Cholesterol, glycerol, octadecadienoic acid, 3-hydroxy-butanoic acid, glucose, isoleucine, valine, leucine, and glycine were notably increased in plasma at weeks 3 and 6 post-surgery compared to levels in rats prior to surgery, and glyceraldehyde 3-phosphate, alanine, and arabinofuranose were significantly decreased at weeks 3 and 6 after surgery.
PLS-DA score plots of serum samples. (A) PLS-DA score plots of OVX rats over the whole experiment (pre-surgery, weeks 3 and 6 post-surgery). (B) PLS-DA score plots of OVX rats and sham rats at 6 weeks post-surgery. The x-axis and y-axis are labeled as PC1 (the first principal component) and PC2 (the second principal component), respectively. One data point stands for one subject: ▪, OVX rats prior to surgery; □, OVX rats at week 6 post-surgery; •, sham rats at week 6 post-surgery; ▴, OVX rats at week 3 post-surgery.
Likewise, a two principal components PLS-DA model was performed between the sham group and the OVX group at week 6 post-surgery. Figure 3B shows the metabolomic movement of both the sham and the OVX groups 6 weeks after bilateral OVX, which could be classified clearly with R2X: 51.2%, R2Y: 99.9%, and Q2Y: 90.7%. In addition, estrogen-deficiency-related modifications of metabolites at different time points are listed in Table 2. The results suggested that estrogen-deficiency-induced obesity greatly affects endogenous metabolite levels compared to the sham group, including a significant increase in the levels of cholesterol, octadecadienoic acid, arachidonic acid, 3-hydroxy-butanoic acid, glutamic acid, glucose, and glycerol and a significant decrease in the levels of glyceraldehyde 3-phosphate, alanine, galactopyranose, and arabinofuranose.
Discussion
Women now, on average, spend more than a third of their life post-menopause. The decline of endogenous estrogen levels, together with physical inactivity, could account for obesity post-menopause, which could lead to an increased risk of cardiovascular diseases (eg, high blood pressure, high blood cholesterol, and high triglyceride levels), diabetes, stroke, and depression in postmenopausal women. Bilateral ovariectomy is the gold standard widely used to simulate postmenopausal obesity in female virgin rats37, 38. Our results showed that rat weight was significantly elevated in the OVX group compared to the sham group. A higher BMI was observed in estrogen-deficient rats beginning the third week post-surgery despite similar food consumption between the sham and OVX groups. Body weight and BMI were negatively correlated with levels of estradiol (E2), indicating that the rat model of estrogen-deficiency-induced obesity was successfully established.
However, the mechanisms of increases in body weight and fat content caused by estrogen deficiency remain unclear. It is generally recognized that rapid decrease in E2 secretion can bring about a hypothalamic-pituitary-ovarian (HPO) axis imbalance, which affects insulin, adrenal cortical hormone, growth hormone, and other hormone metabolism and leads to in vivo glucose and lipid metabolism disorders. These disorders are likely responsible for the pathogenesis of obesity among postmenopausal women. In addition, significantly elevated levels of TC, TG, and LDL-C and slightly reduced levels of HDL-C in the OVX group caused by estrogen-deficiency-induced obesity could indicate high risk for a series of complications.
Estrogen-deficiency-induced obesity is associated with fat accumulation and excessive intake of energy that break down the balance of energy metabolism (Figure 4). Fats are triesters of glycerol and fatty acids. Using the metabolomic approach, glycerol and fatty acid levels (eg, hexadecanoic acid, octadecadienoic acid, oleic acid, and arachidonic acid) were significantly increased in rats with estrogen-deficiency-induced obesity. We presumed that estrogen had a key modulator role in lipid metabolism. Herrero et al reported that myocardial fatty acid utilization was higher in women taking estrogens compared to those not receiving estrogens, which might be attributable to the regulation of β-oxidation of fatty acids39. It has been reported that mRNA expression and the activity of enzymes including medium-chain acyl coenzyme A dehydrogenase and acetyl CoA oxidase, enzymes with fatty acid β-oxidation, are reduced under estrogen deficiency40. Therefore, fatty acid levels may be increased because the estrogen deficiency interferes with the β-oxidation of fatty acids and leads to a reduced decomposition rate of fatty acids. Linoleic acid and essential fatty acids cannot be synthesized by humans and other animals and are only supplied from food components. In our research, linoleic acid was increased 1.4-fold and 1.2-fold compared to pre-surgery levels and to the sham group in the 6th week, respectively. Increased levels of fatty acids might be due to reduced β-oxidation of fatty acids. Moreover, levels of arachidonic acid, a precursor of prostaglandins, thromboxanes, and leukotrienes were elevated in the OVX rats, and arachidonic acid was biosynthesized by linoleic acid catalyzed by a series of enzymes. Therefore, estrogen deficiency might cause a lower rate of β-oxidation of fatty acids and greater biosynthesis of fatty acids. Furthermore, elevated levels of serum glycerol in OVX rats compared to sham rats might be a consequence of self-regulation because obese rats have higher basal lipolytic rates than normal subjects41.
Likewise, glycolysis is regulated by estrogen and estrogen receptors. Furman et al observed that estrogens stimulated the glycolysis process in MCF-7 cells, which could be inhibited by tamoxifen (TAM)42. Using metabolomic analysis, the levels of glyceraldehyde 3-phosphate and alanine were significantly decreased in rats with estrogen deficiency. Glyceraldehyde 3-phosphate, an important metabolic intermediate in glycolysis, is bio-synthesized by phosphoglycerate kinase, which catalyzes the conversion of 1,3-bisphosphoglycerate into glyceraldehyde 3-phosphate, which is the first step of ATP production in glycolysis. A decrease in glyceraldehyde 3-phosphate suggests that estrogen has an adjustment effect in glycolysis, and an estrogen deficiency could interfere with this process. Similar to glyceraldehyde 3-phosphate, the lower alanine levels found in rats with estrogen deficiency would be indirectly correlated with the glycolysis process because disruption of glycolysis leads to pyruvate insufficiency. Moreover, alanine plays a key role in the glucose-alanine cycle between muscle tissue and the liver; glycolysis in the muscle produces pyruvate, which is further converted to alanine. The results of our study are also suggestive of a modified alanine-glucose cycle. In addition, endogenous estrogen regulates skeletal muscle mass in developing rats. Therefore, estrogen loss can alter skeletal muscle recovery from disuse43, 44. As a result, decreased muscle function may also be a reason for the reduction of alanine biosynthesis.
Estrogens are involved in the regulation of not only fatty acid metabolism and glycolysis but also the TCA cycle. The activity of citrate synthase (CS) in skeletal muscle and cerebral blood vessels is enhanced by estrogen therapy in OVX rats45. Raben et al found that β-hydroxyacyl-CoA dehydrogenase (HADH) and CS levels were 20% lower in formerly obese women46. Moreover, pyruvate dehydrogenase, aconitase, and ATP-synthase are regulated by estrogen47. Therefore, more precursor acetyl-CoA may enter into the fatty acid, ketone (eg, acetoacetate, 3-hydroxy-butanoic acid, and acetone), and cholesterol biosynthesis processes as a result of the TCA cycle being attenuated under estrogen deficiency. As a result of the affected metabolic pathway, high levels of cholesterol and fatty acids were observed in OVX rats, and a marked increase in the levels of 3-hydroxy-butanoic acid was observed in rats with bilateral ovariectomy-induced obesity. Furthermore, glycolysis, the TCA cycle, and insulin would decrease the levels of blood glucose in contrast to gluconeogenesis (the generation of glucose from non-carbohydrates), which could increase the levels of blood glucose. In our study, the levels of blood glucose were slightly elevated during obesity in OVX rats, which might be connected with the subdued glycolysis and TCA cycle caused by estrogen deficiency. Insulin resistance resulting from estrogen deficiency is a known risk factor for type 2 diabetes and leads to the high blood glucose levels observed.
Higher cholesterol levels were found in OVX rats compared to sham rats by metabolomic analysis, which was in agreement with the conventional measurements using commercial kits. High blood cholesterol levels in postmenopausal women are a biom arker of obesity due to ovarian hormone deficiency. Mataumoto et al reported that the level of cholesterol 7α-hydroxylase activity, a key enzyme in cholesterol catabolism, was significantly reduced in the liver in OVX rats48. Therefore, excessive acetyl-CoA is used for cholesterol synthesis in OVX rats, but the rate of cholesterol degradation is attenuated because of the decrease in 7α-hydroxylase activity. Obviously, the elevated levels of serum cholesterol were a result of a combination of the above two factors.
The branched-chain aliphatic amino acids (BCAAs), including isoleucine, valine, and leucine, are markedly increased after estrogen deficiency. The BCAAs play important roles in protein synthesis, neurotransmitter synthesis, and branched-chain fatty acid synthesis. They cannot be synthesized by the animal itself and must be supplied by the diet. In our study results, serum amino acid levels were elevated in obesity, which is consistent with previous research49, 50, 51. She et al52 reported that obesity may play a potential role in the regulation of BCAA catabolism. They found that the activities of the BCATm and BCKD enzyme complex, the key enzymes in the first two steps of the BCAAs catabolic pathway, were depressed in obese ob/ob mice and Zucker rats. Therefore, we presumed that the alternations in BCAA metabolism may contribute to increase in BCAAs in obesity. The γ-glutamyl cycle plays a key role in glutathione synthesis, and the transport system for amino acids and glutamic acid, glycine, and cystine take part in the γ-glutamyl cycle. Higher glutamic acid, glycine, and cystine levels in estrogen-deficiency-induced obesity would influence the γ-glutamyl cycle.
Conclusions
Metabolomic profiling with multivariate statistical analysis combined with conventional measurement kit assays indicated a progression of experimental obesity induced by estrogen deficiency in rats. The series of potential biomarkers identified provide fingerprints of rat metabolomic changes during obesity and an overview of multiple metabolic pathways during the progression of obesity that involves glucose metabolism, lipid metabolism, and amino acid metabolism.
Author contribution
Bo MA and Qi ZHANG have been involved in all phases of this project. Guang-ji WANG designed the research. Ji-ye A analyzed the data, and Di WU revised and wrote the paper. Bei CAO, Ying-ying HU, Lin-sheng LIU were involved in measurements. Ying LIU, Yong-lu WANG, and Ya-ya ZHENG were involved in the animal experiments of the project.
References
Haslam DW, James WP . Obesity. Lancet 2005; 366: 1197–209.
Ryan AS, Nicklas BJ, Berman DM . Hormone replacement therapy, insulin sensitivity, and abdominal obesity in postmenopausal women. Diabetes Care 2002; 25: 127–33.
Burke GL, Bertoni AG, Shea S, Tracy R, Watson KE, Blumenthal RS, et al. The impact of obesity on cardiovascular disease risk factors and subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis. Arch Intern Med 2008; 168: 928–35.
Winter Y, Rohrmann S, Linseisen J, Lanczik O, Ringleb PA, Hebebrand J, et al. Contribution of obesity and abdominal fat mass to risk of stroke and transient ischemic attacks. Stroke 2008; 39: 3145–51.
Kirschner MA, Ertel N, Schneider G . Obesity, hormones, and cancer. Cancer Res 1981; 41: 3711–7.
Cleary MP, Grossmann ME, Ray A . Effect of obesity on breast cancer development. Vet Pathol 2010; 47: 202–13.
Cleary MP, Grossmann ME . Minireview: Obesity and breast cancer: the estrogen connection. Endocrinology 2009; 150: 2537–42.
Kuhl H . Breast cancer risk in the WHI study: the problem of obesity. Maturitas 2005; 51: 83–97.
Balog J . Obesity and estrogen. Am J Obstet Gynecol 1978; 130: 242.
de Paz I P, Alameda HC, Olivar RJ . Obesity and menopause. Nutr Hosp 2006; 21: 633–7.
Kanaya AM, Vittinghoff E, Shlipak MG, Resnick HE, Visser M, Grady D, et al. Association of total and central obesity with mortality in postmenopausal women with coronary heart disease. Am J Epidemiol 2003; 158: 1161–70.
Chang CJ, Wu CH, Yao WJ, Yang YC, Wu JS, Lu FH . Relationships of age, menopause and central obesity on cardiovascular disease risk factors in Chinese women. Int J Obes Relat Metab Disord 2000; 24: 1699–704.
Gao Q, Horvath TL . Cross-talk between estrogen and leptin signaling in the hypothalamus. Am J Physiol Endocrinol Metab 2008; 294: E817–26.
Gomori A, Ishihara A, Ito M, Matsushita H, Ito M, Mashiko S, et al. Blockade of MCH1 receptor signalling ameliorates obesity and related hepatic steatosis in ovariectomized mice. Br J Pharmacol 2007; 151: 900–8.
Sorensen MB, Rosenfalck AM, Hojgaard L, Ottesen B . Obesity and sarcopenia after menopause are reversed by sex hormone replacement therapy. Obes Res 2001; 9: 622–6.
Ryan AS, Nicklas BJ, Berman DM . Hormone replacement therapy, insulin sensitivity, and abdominal obesity in postmenopausal women. Diabetes Care 2002; 25: 127–33.
Best PJ, Berger PB, Miller VM, Lerman A . The effect of estrogen replacement therapy on plasma nitric oxide and endothelin-1 levels in postmenopausal women. Ann Intern Med 1998; 128: 285–8.
Manolio TA, Furberg CD, Shemanski L, Psaty BM, O'Leary DH, Tracy RP, et al. Associations of postmenopausal estrogen use with cardiovascular disease and its risk factors in older women. The CHS Collaborative Research Group. Circulation 1993; 88: 2163–71.
Brinton LA, Schairer C . Estrogen replacement therapy and breast cancer risk. Epidemiol Rev 1993; 15: 66–79.
Burry K, Cain JM . Estrogen replacement therapy and risk of ovarian cancer in postmenopausal women. JAMA 2002; 288: 2538; author reply 2539.
Ambrus JL . Postmenopausal hormone therapy increases risk of deep vein thrombosis and pulmonary embolism. Am J Med 1999; 107: 104.
Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA 2004; 291: 2947–58.
Nicholson JK, Lindon JC, Holmes E . 'Metabonomics': understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999; 29: 1181–9.
Nicholson JK, Lindon JC . Systems biology: Metabonomics. Nature 2008; 455: 1054–6.
Nicholson JK, Wilson ID . Opinion: understanding 'global' systems biology: metabonomics and the continuum of metabolism. Nat Rev Drug Discov 2003; 2: 668–76.
Kaddurah-Daouk R, Kristal BS, Weinshilboum RM . Metabolomics: a global biochemical approach to drug response and disease. Annu Rev Pharmacol Toxicol 2008; 48: 653–83.
Saude EJ, Obiefuna IP, Somorjai RL, Ajamian F, Skappak C, Ahmad T, et al. Metabolomic biomarkers in a model of asthma exacerbation: urine nuclear magnetic resonance. Am J Respir Crit Care Med 2009; 179: 25–34.
Chen C, Gonzalez FJ, Idle JR . LC-MS-based metabolomics in drug metabolism. Drug Metab Rev 2007; 39: 581–97.
Zhang Q, Wang GJ, A JY, Wu D, Zhu LL, Ma B, et al. Application of GC/MS-based metabonomic profiling in studying the lipid-regulating effects of Ginkgo biloba extract on diet-induced hyperlipidemia in rats. Acta Pharmacol Sin 2009; 30: 1674–87.
Kamleh MA, Hobani Y, Dow JA, Watson DG . Metabolomic profiling of Drosophila using liquid chromatography Fourier transform mass spectrometry. FEBS Lett 2008; 582: 2916–22.
Lu Y, A J, Wang G, Hao H, Huang Q, Yan B, et al. Gas chromatography/time-of-flight mass spectrometry based metabonomic approach to differentiating hypertension- and age-related metabolic variation in spontaneously hypertensive rats. Rapid Commun Mass Spectrom 2008; 22: 2882–8.
Gu S, A J, Wang G, Zha W, Yan B, Zhang Y, et al. Metabonomic profiling of liver metabolites by gas chromatography-mass spectrometry and its application to characterizing hyperlipidemia. Biomed Chromatogr 2010; 24: 245–52.
Yan B, A J, Wang G, Lu H, Huang X, Liu Y, et al. Metabolomic investigation into variation of endogenous metabolites in professional athletes subject to strength-endurance training. J Appl Physiol 2009; 106: 531–8.
Liu L, Aa J, Wang G, Yan B, Zhang Y, Wang X, et al. Differences in metabolite profile between blood plasma and serum. Anal Biochem 2010; 406: 105–12.
A J, Trygg J, Gullberg J, Johansson AI, Jonsson P, Antti H, et al. Extraction and GC/MS analysis of the human blood plasma metabolome. Anal Chem 2005; 77: 8086–94.
Novelli EL, Diniz YS, Galhardi CM, Ebaid GM, Rodrigues HG, Mani F, et al. Anthropometrical parameters and markers of obesity in rats. Lab Anim 2007; 41: 111–9.
Von Diemen V, Trindade EN, Trindade MR . Experimental model to induce obesity in rats. Acta Cir Bras 2006; 21: 425–9.
Rogers NH, Perfield JW 2nd, Strissel KJ, Obin MS, Greenberg AS . Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 2009; 150: 2161–8.
Herrero P, Soto PF, Dence CS, Kisrieva-Ware Z, Delano DA, Peterson LR, et al. Impact of hormone replacement on myocardial fatty acid metabolism: potential role of estrogen. J Nucl Cardiol 2005; 12: 574–81.
Kamei Y, Suzuki M, Miyazaki H, Tsuboyama-Kasaoka N, Wu J, Ishimi Y, et al. Ovariectomy in mice decreases lipid metabolism-related gene expression in adipose tissue and skeletal muscle with increased body fat. J Nutr Sci Vitaminol (Tokyo) 2005; 51: 110–7.
Wang Y, Sullivan S, Trujillo M, Lee MJ, Schneider SH, Brolin RE, et al. Perilipin expression in human adipose tissues: effects of severe obesity, gender, and depot. Obes Res 2003; 11: 930–6.
Furman E, Rushkin E, Margalit R, Bendel P, Degani H . Tamoxifen induced changes in MCF7 human breast cancer: in vitro and in vivo studies using nuclear magnetic resonance spectroscopy and imaging. J Steroid Biochem Mol Biol 1992; 43: 189–95.
McClung JM, Davis JM, Wilson MA, Goldsmith EC, Carson JA . Estrogen status and skeletal muscle recovery from disuse atrophy. J Appl Physiol 2006; 100: 2012–23.
Sugiura T, Ito N, Goto K, Naito H, Yoshioka T, Powers SK . Estrogen administration attenuates immobilization-induced skeletal muscle atrophy in male rats. J Physiol Sci 2006; 56: 393–9.
Beckett T, Tchernof A, Toth MJ . Effect of ovariectomy and estradiol replacement on skeletal muscle enzyme activity in female rats. Metabolism 2002; 51: 1397–401.
Raben A, Mygind E, Astrup A . Lower activity of oxidative key enzymes and smaller fiber areas in skeletal muscle of postobese women. Am J Physiol 1998; 275: E487–94.
Nilsen J, Irwin RW, Gallaher TK, Brinton RD . Estradiol in vivo regulation of brain mitochondrial proteome. J Neurosci 2007; 27: 14069–77.
Mataumoto J, Kishida T, Ebihara K . Sugar beet fiber suppresses ovarian hormone deficiency-induced hypercholesterolemia in rats. Nutr Res 2001; 21: 1519–27.
Bagdade JD, Bierman EL, Porte D Jr . Are plasma amino acid levels elevated in obesity. N Engl J Med 1970; 282: 166.
Felig P, Marliss E, Cahill GF Jr . Plasma amino acid levels and insulin secretion in obesity. N Engl J Med 1969; 281: 811–6.
Leclercq B, Seve B . Influence of adiposity (genetic or hormonal) on the metabolism of amino acids and nutritional responses. Reprod Nutr Dev 1994; 34: 569–82.
She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ . Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol Endocrinol Metab 2007; 293: E1552–63.
Acknowledgements
This study was supported by a grant from the National Natural Science Foundation of China (Grant No 30873112) and the Six Talent Peek Project of Jiangsu Province (No 07-C-018).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ma, B., Zhang, Q., Wang, Gj. et al. GC-TOF/MS-based metabolomic profiling of estrogen deficiency-induced obesity in ovariectomized rats. Acta Pharmacol Sin 32, 270–278 (2011). https://doi.org/10.1038/aps.2010.196
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2010.196
Keywords
This article is cited by
-
Effects of short- and long-term glucocorticoid-induced osteoporosis on plasma metabolome and lipidome of ovariectomized sheep
BMC Musculoskeletal Disorders (2020)
-
Untargeted metabolomic analysis of the carotenoid-based orange coloration in Haliotis gigantea using GC-TOF-MS
Scientific Reports (2019)
-
Integrative Bone Metabolomics—Lipidomics Strategy for Pathological Mechanism of Postmenopausal Osteoporosis Mouse Model
Scientific Reports (2018)
-
Metabolomic Analysis of Adipose Tissue in Rats Exposed to Triptolide
Chromatographia (2017)
-
Polygonatum stenophyllum improves menopausal obesity via regulation of lipolysis-related enzymes
Journal of Natural Medicines (2016)