Abstract
Aim:
To investigate the role of ATP-sensitive potassium (KATP) channels on blood pressure variability (BPV) in sinoaortic denervated (SAD) rats.
Methods:
SAD was performed on male Sprague-Dawley rats 4 weeks before the study. mRNA expression of Kir6.1, Kir6.2 and SUR2 in aorta and mesenteric artery was determined using real-time quantitative polymerase chain reaction, and confirmed at the protein level using Western blotting and laser confocal immunofluorescence assays. Concentration-response curves of isolated aortic and mesenteric arterial rings to adenosine and pinacidil were established. Effects of KATP channel openers and blocker on BPV were examined in conscious SAD rats.
Results:
Aortic SUR2 expression was significantly greater, while Kir6.1 was lower, in SAD rats than in sham-operated controls. In contrast, in the mesenteric artery both SUR2 and Kir6.1 expression were markedly lower in SAD rats than controls. For both arteries, Kir6.2 expression was indistinguishable between sham-operated and SAD rats. These findings were confirmed at the protein level. Responses of the aorta to both adenosine and pinacidil were enhanced after SAD, while the mesenteric response to adenosine was attenuated. Pinacidil, diazoxide, nicorandil, and glibenclamide significantly decreased BPV.
Conclusion:
These findings indicate that expression of vascular KATP channels is altered by chronic SAD. These alterations influence vascular reactivity, and may play a role in the increased BPV in chronic SAD rats.
Similar content being viewed by others
Introduction
Sinoaortic denervation (SAD), referring to removal of the carotid sinus and aortic arch baroreceptors1, results in baroreflex failure bringing about increase in blood pressure variability (BPV) with 24 h mean blood pressure level unchanged or only slightly increased2, 3. It has been demonstrated that BPV was correlated with target-organ damage independent of mean blood pressure levels in hypertensive subjectives4. More recently the importance of BPV in prediction of risk of vascular events and in accounting for benefits of antihypertensive drugs, especially in prevention of stroke, was emphasized5, 6, 7. Multiple studies have investigated the roles of resting vascular tone changes, arterial pressure levels, the sympathetic nervous system, and vasoactive substances in the increased BPV after SAD3, 8, 9, 10, 11, 12, 13, 14. However, the definite mechanism by which BPV dramatically increase after SAD surgery is still unclear.
ATP-sensitive potassium (KATP) channels are critical regulators of vascular tone, forming a focal point for signaling by many vasoactive transmitters, including adenosine, calcitonin gene-related peptide, noradrenaline, insulin, prostaglandin E1, and arginine vasopressin15, 16, 17, 18, 19. It is therefore a molecular sensor, responding to local metabolic need and vasoactive transmitters, altering smooth muscle contractility and so blood flow to retain homeostasis20, 21, 22. If expression of vascular KATP channels was altered in different blood vessels after SAD surgery, response of KATP channels to vasoactive transmitters would be altered too, which might influence vascular tone and contribute to the increased BPV. Previous studies suggested that persistent high BPV following SAD led to pathological changes, the aorta was most sensitive to increased BPV23, and BPV was mostly determined by resistance vessels8, 9, 11. In this study, we compared the channel expression in aorta and mesenteric artery of sham-operated and SAD rats to demonstrate our hypothesis that expression of vascular KATP channels was altered after SAD surgery. We also examined the effects of KATP channel openers or blocker to assess the role of KATP channels in BPV in chronic SAD rats.
Materials and methods
Animals and drugs
Rats were purchased from the Sino-British SIPPR/BK Lab (Shanghai, China), housed in controlled conditions (temperature: 21±2 °C and lighting: 8:00–20:00) and received a standard rat chow and tap water ad libitum. All surgical and experimental procedures were in accordance with institutional animal care guidelines.
Pinacidil, diazoxide, nicorandil, glibenclamide, phenylephrine and adenosine were purchased from Sigma Chemical Co (St Louis, MO, USA).
Sinoaortic denervation
Sinoaortic denervation (SAD) was performed according to the previously described method1, 12. Briefly, rats were anaesthetized with a combination of ketamine (50 mg/kg, ip) and diazepam (5 mg/kg, ip) and medicated with atropine sulfate (0.5 mg/kg, ip) and procaine benzylpenicillin (60 000 U, im). After a midline neck incision and bilateral isolation of the neck muscles, aortic arch baroreceptor denervation was carried out bilaterally by cutting the superior laryngeal nerves near the vagi, removing the superior cervical ganglia, including a small section of the sympathetic trunk, and sectioning the aortic depressor nerves. The carotid sinus baroreceptors were denervated bilaterally by stripping the carotid bifurcation and its branches, followed by the application of 10% phenol (in 95% ethanol) to the external, internal and common carotid arteries and the occipital artery. The sham operation included the midline neck incision and bilateral isolation of the neck muscles. Animals were allowed to recover spontaneously after surgery. The completeness of SAD was assessed by intravenous injection of phenylephrine (2–5 μg/kg) via the left femoral vein. If a 50 mmHg pressor response to phenylephrine was associated with a bradycardia less than 20 beats/min, the SAD was considered complete. Sham-operated animals exhibited a bradycardia of 60–100 beats/min in response to phenylephrine.
Tissue preparation
Four weeks after SAD or sham-operation, rats were anaesthetized with sodium pentobarbitone (50 mg/kg, ip) and killed by exsanguination. Care was taken to ensure that comparable arteries were examined from sham-operated and SAD rats. The thoracic aorta was carefully dissected from surrounding fat and connective tissue. The mesenteric artery was removed from each rat, and the superior mesenteric artery was located and cleaned of connective tissue until the sixth branch leading to the gut wall was exposed.
Real-time quantitive polymerase chain reaction
Total RNA was extracted from aorta (7 aortas from 7 rats, n=7 for both sham-operated and SAD groups) and mesenteric artery (including the superior mesenteric artery itself until the sixth branch: 21 rats were used per group but 3 arteries were pooled as one sample so that n=7 for both groups) using the TRIzol method (Invitrogen, Carlsbad, CA, USA). With the use of 2 μg total RNA, first strand of cDNA was synthesized by the M-MLV enzyme in a 25 μL reaction mixture (Promega, Madison, WI, USA). With the use of aliquots (2 μL) of reverse transcriptase products, real-time quantitative PCR was performed in a final volume of 20 μL using the gene-specific primers listed in Table 1. Amplification was processed as: 95 °C, 10 s, 1 cycle; 95 °C, 5 s and 60 °C, 20 s for 40 cycles; then melt curve. Gene transcripts were quantified with SYBR Premix Ex Taq Kit (Takara, Otsu, Japan). Data were calculated by the 2−ΔΔCT method, and presented as fold change of transcripts for Kir6.1, Kir6.2, and SUR gene in aortas and mesenteric arteries of SAD rats compared with sham-operated group (defined as 1.0-fold). Rat β-actin was used as an internal control. The relative expression of the target gene was normalized to the β-actin level.
Western blotting
Protein level of Kir6.1, Kir6.2, and SUR2 of both aorta (6 rats were used per group but 2 aortas were pooled as one sample so that n=3 for both groups) and mesenteric artery (including the superior mesenteric artery itself until the sixth branch: 15 rats were used per group but 5 arteries were pooled as one sample so that n=3 for both group) from sham-operated and SAD rats were determined. Tissues were washed with ice-cold phosphate-buffered saline (PBS), and then homogenized with ice-cold lysis buffer (20 mmol/L Tris-HCl, pH 7.4, 2.5 mmol/L EDTA, 1% Triton X-100, 10% glycerol, and 1 mmol/L PMSF, 1 mmol/L DTT, 10 mg/mL leupeptin), and the supernatant was obtained by centrifugation at 11 000×g for 15 min at 4 °C. Samples were separated on 8% SDS-PAGE gels, transferred to a polyvinylidene difluoride (PVDF) membrane, and blocked by 3 h incubation at 25 °C in TBST (Tris-buffered saline with 0.1% Tween 20, pH 7.4) plus 10% skimmed milk powder. The PVDF membrane was incubated overnight at 4 °C with primary antibodies for Kir6.1 (1:200; Alomone Labs Ltd, Jerusalem, Israel), Kir6.2 (1:200; Alomone Labs Ltd) or SUR2 (1:200; Santa Cruz, CA, USA). PVDF membranes were washed for 10 min 3 times with TBST and incubated with secondary antibodies conjugated with horseradish peroxidase. The bound antibody was visualized on Kodak Biomax film (Eastman Kodak, Rochester, NY, USA) using a Pierce Supersignal substrate chemiluminescence detection kit. Results from these analyses were quantified through densitometry.
Confocal imaging
Frozen sections (4–8 μm) of both aortas and superior mesenteric arteries were blocked in 3% normal goat serum/0.3% Triton X-100/0.1% Bovine serum albumin (BSA) in PBS (room temperature for 1 h), followed by incubation with either rabbit anti-kir6.1 (1:100), rabbit anti-Kir6.2 (1:100), or goat anti-SUR2 (1:200) at 4 °C overnight. Subsequently, the sections were incubated with the affinity-purified second antibody, goat anti-rabbit or rabbit anti-goat fluorescein isothiocyanate (FITC) antibody (1:200; Sigma Chemical Co, St Louis, MO, USA). Sections were then rinsed, dried, and coverslipped with Dako fluorescence mounting medium. Laser scanning confocal microscopy was performed with the use of a Leica TCS SP2 system (Leica, Wetzlar, Germany). The objective was ×20. FITC was excited at 488 nm and emission was detected at 500–600 nm. The imagines were analyzed with Leica Confocal software.
Vascular reactivity
To determine if vascular reactivity to adenosine (an endogenous vasoactive substance, relaxing vascular smooth muscle cells by opening KATP channels20) and pinacidil (a nonselective opener of KATP channels) was altered in chronic SAD rats. 5 mm long thoracic aortic rings (17–21 aortic rings obtained from 11 rats for both the sham and SAD groups) or superior mesenteric arterial rings (12 superior mesenteric arterial rings from 9 rats for both the sham and SAD groups) were prepared for assessment of vascular reactivity (IOX software, EMKA Technology, Inc., Paris, France). Resting tension was 2.5 g or 0.8 g respectively, and contraction was induced by phenylephrine (3×10-7 mol/L). The relaxant effects of adenosine (10-7 to 3×10-4mol/L) and pinacidil (10-8 to 3×10-5mol/L for aorta, 10-8 to 3×10-6mol/L for superior mesenteric artery) were assessed.
Blood pressure measurement
To assess the role of KATP channels in BPV in chronic SAD rats, the effects of KATP channel openers or blocker on BPV were examined 4 weeks after the surgery. Four groups (n=8–10 for each group) were designed: 1) pinacidil, 2 mg/kg; 2) diazoxide, 20 mg/kg; 3) nicorandil, 10 mg/kg; 4) glibenclamide, 20 mg/kg. Blood pressure was recorded by a computerized system (MPA 2000M, Alcott Biotech Co LTD, Shanghai, China) using a previously described method12. Two 60-min sampling periods were programmed. Before the beginning of first period, isotonic saline was administered intravenously in a volume of 3 mL/kg over a 5-min period. After a 15-min equilibration period, arterial pressure was then recorded for 60-min. Before the beginning of the second period, one of the drug treatments listed above was given as a 5-min infusion, and after a 15-min equilibration period data were recorded for 60-min. The final 30-min of each 60-min period was used for analysis. Systolic and diastolic BP and heart period were averaged beat-to-beat during the 30-min test period. BPV was defined as the standard deviation of beat-to-beat systolic or diastolic BP over the 30-min analysis period.
Statistical analyses
Data are presented as mean±SEM. Differences in both mRNA and protein between sham-operated and SAD group were analyzed using Student's un-paired t-test. Differences in vascular reactivity between sham-operated and SAD group were analyzed by repeated measures analysis of variance. Changes of BPV, BP and heart period before and after drug infusion were analyzed by paired t-tests. Two-tailed P<0.05 was considered statistically significant.
Results
Presence of Kir6.1, Kir6.2, and SUR2
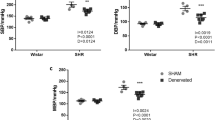
SUR2 mRNA expression was significantly greater in the aorta of SAD rats (1.67±0.370) compared with controls (1.00±0.084; n=7, P<0.05). In contrast, aortic Kir6.1 mRNA expression was lower in SAD rats than controls (0.67±0.052 vs 1.00±0.095, n=7, P<0.05). In the mesenteric artery both SUR2 (0.45±0.084 vs 1.00±0.141, n=7, P<0.05) and Kir6.1 expression (0.76±0.047 vs 1.00±0.049, n=7, P<0.05) were lower in SAD rats than in controls. No significant differences were observed in Kir6.2 expression between sham-operated and SAD rats for both kinds of arteries. Results of protein analysis by Western blotting were in agreement with the mRNA expression (Figure 1). Confocal imaging using fluorescence-labeled antibodies to Kir6.1, Kir6.2 and SUR2 localized these proteins in both kinds of arteries, as predicted by the western blot results (Figure 2). These results demonstrate that in chronic SAD rats KATP channels are altered when compared with the controls, that is, expression of both Kir6.1 and SUR2 are significantly reduced in mesenteric artery; decrease in Kir6.1 expression accompanied with increase in SUR2 expression is found in aorta.
Expression of Kir6.1, Kir6.2 and SUR2 in both aorta and mesenteric artery in sinoaortic denervated and sham-operated rats. A and B, fold changes of mRNA level (Kir6.1, Kir6.2, and SUR2) in aorta and mesenteric artery (n=7). C and D, fold changes of protein level (Kir6.1, Kir6.2, and SUR2) in aorta and mesenteric artery (n=3). The representative images are from three independent experiments. Data are expressed as mean±SEM. bP<0.05, cP<0.01 vs sham-operated rats by unpaired Student's t-test.
Representative images of Kir6.1, Kir6.2, and SUR2 expression in aorta (A–C) and mesenteric artery (D–F) from sinoaortic denervated (SAD) and sham-operated rats using laser scanning confocal microscopy. Aortic SUR2 expression was greater, while Kir6.1 was lower, in SAD rats than in sham-operated controls (A and C). In the mesenteric artery both SUR2 and Kir6.1 expression were markedly lower in SAD rats than controls (D and F). For both arteries, Kir6.2 expression was indistinguishable in sham-operated compared with SAD rats (B and E). Similar images were observed in vessels from at least 3 rats for each group. FITC,fluorescein isothiocyanate; DIC, differential interference contrast. The objective was ×20, scale bar: 50 μm.
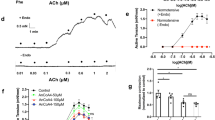
Vasodilatation induced by adenosine and pinacidil
In phenylephrine-contracted, endothelium-intact aortic rings, both adenosine and pinacidil induced concentration-dependent relaxation. Compared to the sham-operated group, the relaxation response of the aorta to both adenosine and pinacidil was significantly enhanced in SAD rats (Figure 3A and 3B); the relaxation response of the mesenteric artery to adenosine was obviously attenuated (Figure 3C). However, pinacidil did not induce relaxation differences between the sham operated and SAD group. These data suggest that vasodilatation by opening KATP channels may depend on the level SUR2 expression.
Vasodilatation induced by adenosine and pinacidil in phenyl-ephrine-precontracted intact rat aorta (A, B) and mesenteric artery (C, D). Relaxant responses are expressed as the percentage reduction in tension produced by phenylephrine. Each point represents mean±SEM. (thoracic aorta, 17–21 aortic rings obtained from 9–12 rats; superior mesenteric artery, 12 superior mesenteric arterial rings from 9–12 rats). Sham, sham operation. cP<0.01 vs sham-operated rats by repeated measures analysis of variance.
Blood pressure induced by KATP channel openers and blocker
Table 2 summarizes the effects of pinacidil, diazoxide, nicorandil, and glibenclamide on systolic and diastolic BP and heart period in SAD rats. The KATP channel openers, pinacidil, diazoxide and nicorandil, significantly decreased systolic and diastolic BP, and slightly but significantly shortened heart period. The KATP channel blocker, glibenclamide, significantly increased BP but did not obviously alter heart period.
Blood pressure variability induced by KATP channel openers and blocker
The effects of the different KATP channel openers and blocker on BPV in SAD rats are shown in Figure 4. Pinacidil and diazoxide reduced SBPV by 40%±4.9% or 34%±6.1%, and DBPV by 40%±5.4% or 38%±6.8% (Figure 4A and 4B). Nicorandil had a similar effect, reducing SBPV by 45%±5.3% and DBPV by 45%±4.7% (Figure 4C). The KATP channel blocker glibenclamide also significantly decreased SBPV by 35%±7.0% and DBPV by 38%±8.3% (Figure 4D), despite the fact that this agent increased resting BP whereas pinacidil, diazoxide and nicorandil reduced it (Table 2). These results that either opening or blocking KATP channels reduced BPV suggest that KATP channels play a role in the increased BPV in chronic SAD rats.
Effects of KATP channel openers and blocker on blood pressure variability (BPV) in sinoaortic denervated (SAD) rats. Pinacidil (n=10), diazoxide (n=8), nicorandil (n=8), and glibenclamide (n=10) significantly decreased BPV. SBPV, systolic BPV; DBPV, diastolic BPV. Data are expressed as mean±SEM. cP<0.01 vs isotonic saline by paired t-test.
Discussion
The current study provides two new findings. Firstly, we demonstrate that expression of vascular KATP channels is altered by chronic SAD. The SUR2 subunit was upregulated in the aorta but downregulated in the mesenteric artery. Consistent with this finding, vasodilator responses to KATP channel opener pinacidil were enhanced in the aorta of SAD rats but blunted in the mesenteric artery. Thus, chronic SAD results in changes in the expression and function of KATP channels that differ according to vascular territory. Our second major finding was that either opening or blocking KATP channels decreased BPV in SAD rats. We interpret these findings to indicate that KATP channels with a functional state play a role in enhanced BPV in chronic SAD rats.
KATP channels are hetero-octamers consisting of 4 sulfonylurea receptors (SUR) interacting with 4 channel subunits (Kir channels)21. The major isoform of the KATP channel in vascular muscle cells is composed of Kir6.1 and SUR2B subunits24, 25. In the vasculature, KATP channels provide a background potassium conductance critical for regulation of arterial tone in response to local metabolic need and vasoactive factors15, 26, and operate as molecular sensors of cellular metabolism21. Genetic disruption of the abcc9 (SUR2) gene leads to coronary artery vasospasm and raises resting BP27. The function and/or expression of the KATP channels appears to be altered under a range of circumstances such as diabetes mellitus28, 29, myocardial ischemia30, and hypertension31, 32. We hypothesized that expression of KATP channels is altered in chronic SAD rats.
In the present study, the impact of chronic SAD on the expression of vascular KATP channels was examined. We determined the expression of SUR2, Kir6.1, and Kir6.2 of both the aorta and mesenteric artery. In SAD rats, aortic expression of SUR2 was significantly greater than that in control rats while Kir6.1 expression was lower. In the case of mesenteric artery both SUR2 and Kir6.1 expression were markedly lower in SAD rats than controls. No significant differences in Kir6.2 expression were observed between SAD and sham-operated rats for both kinds of arteries. These results show that expression of vascular KATP channels is altered after SAD surgery. The factors leading to these alterations in chronic SAD remain to be determined, but could include enhanced sympathetic drive8, 33, haemodynamic variability9, and adaptive changes in the function of local vasoactive substances11, 20 after loss of baroreflex control of peripheral resistance.
K+ channel-opener drugs activate KATP channels by interacting with the SUR subunit21. Adenosine, a metabolite of ATP, acts on adenosine A2A receptors in vascular smooth muscle cell to cause dilatation by opening KATP channels20. In our work, both pinacidil and adenosine were used to assess vascular reactivity in sham-operated and SAD rats. We found that responses of the aorta to both adenosine and pinacidil were enhanced 4 weeks after SAD, while the mesenteric responses to adenosine were attenuated. Thus, changes in SUR2 expression appear to translate into changes in vascular function, this seems consistent with the idea suggested by Farzaneh et al34: in Kir6.1/SUR2, it is the SUR that underlies the metabolic sensitivity. It is tempting to speculate that such changes in SUR2 expression in the vessels may also be present in the microvasculature, and so lead to changes in the control of peripheral resistance and perhaps BPV.
Blood pressure variability has important physiological and clinical implications4, 5, 6, 7, 35, 36. The most simple and commonly held explanation for the rise in BPV after SAD is the loss of the regulatory feedback function of the baroreceptor reflex. However, much remains to be understood concerning the dramatic increase in BPV after SAD1, 2, 3. Alper et al8 suggested that instability of BP was related to fluctuation in sympathetic vasomotor tone. Julien et al33 suggested that the sympathetic nervous system might directly generate part of the lability of BP in SAD rats. It has also been suggested that endogenous vasoconstrictors, by affecting the level of vascular tone, increased BPV11. Thus, basal vascular tone may be critical for the generation of BPV associated with SAD. These observations suggest that regulation of BPV in SAD rats is rather complex.
KATP channels, working as molecular sensors of cellular metabolism21, regulate vascular tone in response to endogenous substances, such as adenosine, noradrenaline and vasopressin15, 16, 17, 18, 19. We hypothesized that KATP channels contribute to the BPV after SAD. To test this hypothesis we examined the impact, on BPV in the SAD rat, of eliminating the ability of vasoactive substances signaling through KATP channels. To interfere with KATP channel signaling we administered KATP channel openers or blocker. We found that pinacidil (a nonselective opener of KATP channels) and diazoxide (being able to open most of the KATP channels except the cardiac sarcolemmal KATP channels), and nicorandil (the vascular relevant opener) all significantly decreased BPV in SAD rats. To further investigate our hypothesis, the KATP channel blocker glibenclamide (a nonselective blocker of KATP channels) was used. BPV was significantly decreased even though this agent had the opposite effect on basal BP (increased) to the openers (reduced). Thus, either opening or blocking KATP channels decreases BPV in SAD rats, support our idea that KATP channels play a role in the increased BPV in chronic SAD rats.
In summary, this study showed that both expression and function of vascular KATP channels was altered by chronic SAD, and either opening or blocking KATP channels decreased BPV in SAD rats. These data support the concept that vascular KATP channels play a role in the increased BPV in chronic SAD rats.
Author contribution
Fu-ming SHEN, Ding-feng SU, and Yi-li YANG designed the research; Zhong-wei YANG, Dong-jie LI, Chong LIU, and Ping HAN performed the research and analyzed the data; Zhong-wei YANG, and Dong-jie LI wrote the paper.
References
Krieger EM . Neurogenic hypertension in the rat. Circ Res 1964; 15: 511–21.
Norman RA Jr, Coleman TG, Dent AC . Continuous monitoring of arterial pressure indicates sinoaortic denervated rats are not hypertensive. Hypertension 1981; 3: 119–25.
Pires SL, Julien C, Chapuis B, Sassard J, Barres C . Spontaneous renal blood flow autoregulation curves in conscious sinoaortic baroreceptor-denervated rats. Am J Physiol Renal Physiol 2002; 282: F51–8.
Tatasciore A, Renda G, Zimarino M, Soccio M, Bilo G . Parati G, et al. Awake systolic blood pressure variability correlates with target-organ damage in hypertensive subjects. Hypertension 2007; 50: 325–32.
Rothwell PM . Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet 2010; 375: 938–48.
Rothwell PM, Howard SC, Dolan E, O'Brien E, Dobson JE, Dahlof B, et al. Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol 2010; 9: 469–80.
Webb AJ, Fischer U, Mehta Z, Rothwell PM . Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet 2010; 375: 906–15.
Alper RH, Jacob HJ, Brody MJ . Regulation of arterial pressure lability in rats with chronic sinoaortic deafferentation. Am J Physiol 1987; 253: H466–74.
Trapani AJ, Barron KW, Brody MJ . Analysis of hemodynamic variability after sinoaortic denervation in the conscious rat. Am J Physiol 1986; 251: R1163–9.
Jacob HJ, Alper RH, Brody MJ . Lability of arterial pressure after baroreceptor denervation is not pressure dependent. Hypertension 1989; 14: 501–10.
Jacob HJ, Alper RH, Grosskreutz CL, Lewis SJ, Brody MJ . Vascular tone influences arterial pressure lability after sinoaortic deafferentation. Am J Physiol 1991; 260: R359–67.
Shen FM, Su DF . The effect of adenosine on blood pressure variability in sinoaortic denervated rats is mediated by adenosine A2a-receptor. J Cardiovasc Pharmacol 2000; 36: 681–6.
Pires SL, Barres C, Sassard J, Julien C . Renal blood flow dynamics and arterial pressure lability in the conscious rat. Hypertension 2001; 38: 147–52.
Stauss HM, Petitto CE, Rotella DL, Wong BJ, Sheriff DD . Very low frequency blood pressure variability is modulated by myogenic vascular function and is reduced in stroke-prone rats. J Hypertens 2008; 26: 1127–37.
Quayle JM, Nelson MT, Standen NB . ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev 1997; 77: 1165–232.
Tan JH, Al Abed A, Brock JA . Inhibition of KATP channels in the rat tail artery by neurally released noradrenaline acting on postjunctional alpha2-adrenoceptors. J Physiol 2007; 581: 757–65.
Yasui S, Mawatari K, Kawano T, Morizumi R, Hamamoto A, Furukawa H, et al. Insulin activates ATP-sensitive potassium channels via phosphatidylinositol 3-kinase in cultured vascular smooth muscle cells. J Vasc Res 2008; 45: 233–43.
Eguchi S, Kawano T, Yinhua, Tanaka K, Yasui S, Mawatari K, et al. Effects of prostaglandin E1 on vascular ATP-sensitive potassium channels. J Cardiovasc Pharmacol 2007; 50: 686–91.
Shi W, Cui N, Shi Y, Zhang X, Yang Y, Jiang C . Arginine vasopressin inhibits Kir6.1/SUR2B channel and constricts the mesenteric artery via V1a receptor and protein kinase C. Am J Physiol Regul Integr Comp Physiol 2007; 293: R191–9.
Bryan PT, Marshall JM . Cellular mechanisms by which adenosine induces vasodilatation in rat skeletal muscle: significance for systemic hypoxia. J Physiol 1999; 514: 163–75.
Nichols CG . KATP channels as molecular sensors of cellular metabolism. Nature 2006; 440: 470–6.
Colantuono G, Tiravanti EA, Di Venosa N, Cazzato A, Rastaldo R, Cagiano R, et al. Hyperoxia confers myocardial protection in mechanically ventilated rats through the generation of free radicals and opening of mitochondrial ATP-sensitive potassium channels. Clin Exp Pharmacol Physiol 2008; 35: 64–71.
Miao CY, Su DF . The importance of blood pressure variability in rat aortic and left ventricular hypertrophy produced by sinoaortic denervation. J Hypertens 2002; 20: 1865–72.
Li L, Wu J, Jiang C . Differential expression of Kir6.1 and SUR2B mRNAs in the vasculature of various tissues in rats. J Membr Biol 2003; 196: 61–9.
Shi Y, Cui N, Shi W, Jiang C . A short motif in Kir6.1 consisting of four phosphorylation repeats underlies the vascular KATP channel inhibition by protein kinase C. J Biol Chem 2008; 283: 2488–94.
Suzuki M, Li RA, Miki T, Uemura H, Sakamoto N, Ohmoto-Sekine Y, et al. Functional roles of cardiac and vascular ATP-sensitive potassium channels clarified by Kir6.2-knockout mice. Circ Res 2001; 88: 570–7.
Chutkow WA, Pu J, Wheeler MT, Wada T, Makielski JC, Burant CF, et al. Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 KATP channels. J Clin Invest 2002; 110: 203–8.
Miura H, Wachtel RE, Loberiza FR Jr, Saito T, Miura M, Nicolosi AC, et al. Diabetes mellitus impairs vasodilation to hypoxia in human coronary arterioles: reduced activity of ATP-sensitive potassium channels. Circ Res 2003; 92: 151–8.
Fan LH, Tian HY, Ma AQ, Hu Z, Huo JH, Cao YX . Altered ATP-sensitive potassium channels may underscore obesity-triggered increase in blood pressure. Acta Pharmacol Sin 2008; 29: 1167–74.
Akao M, Otani H, Horie M, Takano M, Kuniyasu A, Nakayama H, et al. Myocardial ischemia induces differential regulation of KATP channel gene expression in rat hearts. J Clin Invest 1997; 100: 3053–9.
Ghosh M, Hanna ST, Wang R, McNeill JR . Altered vascular reactivity and KATP channel currents in vascular smooth muscle cells from deoxycorticosterone acetate (DOCA)-salt hypertensive rats. J Cardiovasc Pharmacol 2004; 44: 525–31.
Shimokawa J, Yokoshiki H, Tsutsui H . Impaired activation of ATP-sensitive K+ channels in endocardial myocytes from left ventricular hypertrophy. Am J Physiol Heart Circ Physiol 2007; 293: H3643–9.
Julien C, Zhang ZQ, Barres C . Role of vasoconstrictor tone in arterial pressure lability after chronic sympathectomy and sinoaortic denervation in rats. J Auton Nerv Syst 1993; 42: 1–10.
Farzaneh T, Tinker A . Differences in the mechanism of metabolic regulation of ATP-sensitive K+ channels containing Kir6.1 and Kir6.2 subunits. Cardiovasc Res 2008; 79: 621–31.
Wu MY, Ma XJ, Yang C, Tao X, Liu AJ, Su DF, et al. Effects of allisartan, a new AT1 receptor blocker, on blood pressure and end-organ damage in hypertensive animals. Acta Pharmacol Sin 2009; 30: 307–13.
Wang JL, Wang L, Wu ZT, Yuan WJ, Su DF, Ni X, et al. Low dose of moxonidine within the rostral ventrolateral medulla improves the baroreflex sensitivity control of sympathetic activity in hypertensive rat. Acta Pharmacol Sin 2009; 30: 1594–600.
Acknowledgements
This work was supported in part by the grants from National Natural Science Foundation of China (81070118), Shanghai Municipal Education Commission (10ZZ52), and the National Basic Research Program of China (2009CB521900).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Zw., Li, Dj., Liu, C. et al. Role of vascular KATP channels in blood pressure variability after sinoaortic denervation in rats. Acta Pharmacol Sin 32, 194–200 (2011). https://doi.org/10.1038/aps.2010.195
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2010.195
Keywords
This article is cited by
-
The baroreflex afferent pathway plays a critical role in H2S-mediated autonomic control of blood pressure regulation under physiological and hypertensive conditions
Acta Pharmacologica Sinica (2021)
-
TRPC6 participates in the development of blood pressure variability increase in sino-aortic denervated rats
Heart and Vessels (2020)
-
Metformin improves the angiogenic functions of endothelial progenitor cells via activating AMPK/eNOS pathway in diabetic mice
Cardiovascular Diabetology (2016)