Abstract
Aim:
To evaluate whether insulin intervention could affect the metabolic and endocrine functions of adipose tissue.
Methods:
C57BL/6 mice were fed on a high-fat-diet for 12−16 weeks to induce insulin resistance. Insulin intervention was administered in the high-fat-diet mice for 4 weeks at 12 weeks (early insulin treatment) or 16 weeks (late insulin treatment). Intraperitoneal glucose tolerance tests were performed before and after insulin treatment. Expression levels of factors involved in the triglyceride synthesis and endocrine functions of adipose tissue including phosphoenolpyruvate carboxykinase (PEPCK-C), fatty acid synthase (FAS), aquaporin 7 (AQP7), adiponectin, visfatin, and interleukin-6 (IL-6) were determined by Western blot.
Results:
In the obese mice, glucose tolerance was impaired; triglyceride content was increased in the liver tissue; protein expression of FAS and adiponectin was decreased; expression of visfatin was increased in adipose tissue. After 4-week insulin treatment, glucose tolerance was improved; triglyceride content was decreased in the liver and skeletal muscle; expression of PEPCK-C, FAS, and adiponectin was increased in the adipose tissue; IL-6 and AQP7 expression was reduced in the fat. Early insulin treatment had better effect in increasing the expression of FAS and PEPCK-C and decreasing the expression of IL-6.
Conclusion:
These results indicate that insulin can target adipocytes for improvement of insulin sensitivity through stimulating triglyceride synthesis and partly improving endocrine functions.
Similar content being viewed by others
Introduction
Early intensive insulin treatment has beneficial effect in the treatment of type 2 diabetes. However, the mechanism of such effect of insulin remains to be investigated. Previous studies including our clinical trial have shown that early insulin intervention significantly prolonged control of hyperglycemia and improved both beta cell function and insulin sensitivity1, 2, 3. However, there is still doubt as to whether exogenous insulin usage could exacerbate hyperinsulinemia in diabetic patients, which might further damage insulin sensitivity.
In addition to the muscle and liver tissue, adipose tissue is the third metabolically relevant site of insulin action. More and more studies have emerged to show that adipose tissue might be the primary defect leading to insulin resistance elsewhere4. It is known that insulin is the primary hormone in the stimulation of synthesis and storage of triglyceride (TG) in adipocytes and disarrangement in these functions of adipose tissue may result in increased release of FFA and glycerol into circulation, ultimately inducing insulin resistance through lipotoxicity in skeletal muscle and liver. Correction of these dysfunctions by thiazolidinediones (TZDs) has been shown to improve the whole-body insulin sensitivity5, 6. In adipocytes, TG synthesis is dependent on glycerol and fatty acids, which generated from glucose by enzymes, such as phosphoenolpyruvate carboxykinase (PEPCK-C), and fatty acid synthase (FAS). PEPCK-C catalyzes glyceroneogenesis and FAS promotes fatty acids synthesis. Aquaporin 7 (AQP7) is a sole glycerol channel in adipocytes and is expressed abundantly in adipose tissue7. AQP7 deficiency has been shown to be associated with obesity8.
In addition to storage function, adipose tissue has endocrine activities. Adiponectin, visfatin, and interleukin-6 (IL-6) etc, are secreted by adipose tissue and involved in the regulation of energy homeostasis, insulin sensitivity, lipid metabolism, inflammation, and immunity. Dysregulation of these hormones/adipokines is emerging as an important mechanism of systemic insulin resistance and metabolic disease9. Insulin infusion was shown to influence the secretion of adiponectin and IL-610. The effect of insulin on visfatin expression in adipose tissue remains to be determined.
In this present study, we explored the effects of insulin on adipose tissue in a chronic study, focusing on the metabolic and endocrine functions. Expression of these factors in adipose tissue was examined in dietary obese C57BL/6 mice after four weeks treatment with insulin. The result suggests that insulin stimulates TG synthesis and partly improves endocrine functions in this mouse model of insulin resistance.
Materials and methods
Reagents
Primary antibodies to PEPCK-C and visfatin were purchased from Santa Cruz Biotechnology, Inc (USA). Primary antibodies to adiponectin and IL-6 were purchased from Chemicon international, Inc (USA). Primary antibody to AQP7 was obtained from Abcam Ltd (UK) and primary antibody to FAS was purchased from Cell Signaling Technology, Inc (USA). Primary antibody for β-actin and all the second antibodies were purchased from Boster Ltd (China).
Animals and intervention
C57BL/6 mice (40) were purchased at 5-weeks in age from Shanghai SLAC Laboratory Animal CO LTD (Shanghai Institute for Biological Sciences, China), and were maintained in pathogen-free barrier conditions at animal facility of Sun Yat-sen University. Mice were housed individually at room temperature 23±1 °C with a 12-h day-night light cycle (lights on at AM 8:00), and had free access to water and diet. After quarantine for 1 week, the mice were divided into four groups (10 per group): (1) a lean control (NC) group, fed on chow diet (64% carbohydrate, 10% fat, and 26% protein); (2) high-fat-diet (HFD) control (FC) group, fed on HFD (28% carbohydrate, 60% fat, and 12% protein); (3) early insulin intervention (INS1) group, fed on HFD and treated with insulin at 12 weeks; (4) late insulin intervention (INS2) group, fed on HFD and treated with insulin at 16 weeks. The mice were on HFD for 12 weeks, except for INS2 group, which received HFD for 16 weeks, while mice in NC group continued to consume the standard chow diet. The insulin treatment was administered with Glargine for 4 weeks through daily subcutaneous injection at a dosage of 0.5–0.75 IU (Sanofi-Aventis, France). Blood glucose (BG) was determined in the tail vein blood of mice every 3 d to adjust insulin dosage to maintain BG levels in the same range as the lean control mice. In the INS2 group, the insulin intervention began at 16 weeks on HFD. The mice in NC and FC groups received vehicle injection daily. Intraperitoneal glucose tolerance tests (IPGTT) were performed just before and after the intervention. All procedures were approved by the Sun Yat-sen University Animal Care and Use Committee with NIH standards.
IPGTT
After 6 h of fasting, mice were weighted and injected with glucose at 1.5 mg/g body weight. Blood glucose was determined with a Glucometer (Johnson & Johnson, USA) at 0, 15, 30, and 90 min post injection.
Plasma and tissue sampling
At the end of experiment, mice were euthanized under anesthetizer with an intraperitoneal injection of chloral hydrate. Blood samples were collected and stored at −80 °C. Liver, gastrocnemius muscle, and visceral fat (VF) were isolated and immediately frozen in liquid nitrogen and stored at −80 °C. The liver and VF (including omental fat, epididymis fat, and retroperitoneal fat) were weighed.
TG content in liver and muscle tissue
About 200 mg liver and muscle tissue were homogenized in ice cold buffer (2 mmol/L NaCl, 20 mmol/L EDTA, 50 mmol/L PBS, pH 7.4). Protein concentrations were determined by bicinchoninic acid (BCA) protein assay (Pierce Chemical Co, USA). Tertbutyl alcohol (10 μL) and 5 μL of Triton X-100 and methanol mixture (1:1, v/v) were added into 10 μL homogenate. After centrifugation for 5 min at 4000 r/min, the supernatant was measured for TG concentration with enzymatic method (Human Gesellschaft für Biochemica und Diagnostica mbH, Germany).
Western blot
VF tissue (about 200 mg) was homogenized in ice-cold buffer. Protein concentrations were determined using a BCA protein assay (Pierce Chemical, USA). Protein of 50 μg was loaded in each well and resolved on 10% SDS-PAGE, then transferred onto polyvinylidene difluoride (PVDF) membranes (0.45 μm; Millipore, USA) at 4 °C. The membranes were preblotted in 5% skim milk Tris-buffer (TBS) with 0.1% Tween 20 (TBST) for 2 h at room temperature and then blotted with primary antibody at 1:500 dilution overnight at 4 °C. After being washed four times (10 min/time) in TBST buffer at room temperature, the membrane was incubated with the second antibody for 2 h and then treated for chemiluminescence (Santa Cruz, USA). The intensity of target proteins and reference protein were quantified using Gene Tool software and the relative gray level of target proteins over reference protein was used to represent the signal strength for each protein.
Blood chemistry
Fasting blood glucose (FBG) was determined using Lifescan One touch glumeter (Johnson & Johnson, USA). Total cholesterol (TC) and TG were measured by enzymatic colorimetric assay (Human Gesellschaft für Biochemica und Diagnostica mbH, Germany). Fasting free fatty acid (FFA) was measured by chromatometry (Daiichi Pure Chemicals Co, Ltd, Japan).
Statistical analysis
All data were expressed as mean±SEM. The significance of group differences was evaluated using ANOVA for multiple comparisons. The least-square deconvolution (LSD) was performed to detect differences between two groups. Statistical significance was set at P<0.05 (two tails).
Results
Metabolic and biochemical parameters
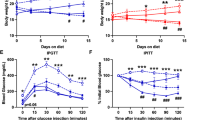
On HFD, the body weights were significantly increased in mice after 4 weeks (P<0.05) (Figure 1A). After 12 weeks on HFD, the mice were divided into three groups, including fat control (FC), early insulin treatment (INS1) and late insulin treatment (INS2). During insulin treatment, the mice remained on HFD. The body weights of these three groups were not significantly different (data not shown).
Metabolic parameters among groups. (A) Body weight of C57BL/6 mice given a high-fat diet or a norma diet. bP<0.05, cP<0.01 vs NC group. (B) Intraperitoneal glucose tolerance tests (IPGTT) performed before intervention (IPGTT1). bP<0.05 vs NC group. (C) IPGTT performed after different intervention (IPGTT2). bP<0.05 vs NC group; eP<0.05 vs FC group. (D) Comparison in fasting TC level among groups. (E) Comparison in fasting TG level among groups. (F) Comparison in fasting FFA level among groups. (G) Comparison in TG content in the liver tissue among groups. (H) Comparison in TG content in the muscle tissue among groups.
At 12 weeks on HFD, IPGTT was performed in the mice (Figure 1B). The fasting blood glucose was not increased by HFD at 12 weeks. However, the blood glucose was significantly increased in the obese mice at 30 and 90 min after glucose injection (P<0.05). The increase was consistent in the three groups of obese mice (FC, INS1, and INS2). After insulin intervention, IPGTT was conducted again in the mice (Figure 1C). The fasting blood glucose was not changed by insulin. However, glucose tolerance was improved in INS1 and INS2 groups. At 15, 30, and 90 min after glucose injection, blood glucose levels were lower in INS1 and INS2 groups than those of FC group (P<0.05). These results suggested that insulin therapy for 4-week improved glucose tolerance in dietary obese mice.
Compared with the FC group, the FFA level in INS1 group was reduced (P<0.01) (Figure 1F). The high-fat-diet increased TG accumulation in the liver of C57BL/6 mice compared with the NC group (P<0.01). The increase was reduced by 4-week insulin intervention in the INS1 and INS2 groups (P<0.05). Insulin treatment also decreased TG content in muscle tissue of mice (Figure 1G, 1H) (P<0.01, INS1 vs FC; P<0.05, INS2 vs FC).
FAS, PEPCK-C, and AQP7 expression
Compared with the lean control (NC group), the obese group had reduction in protein levels of FAS in adipose tissue (P<0.01). The reduction was reversed by the insulin therapy (P<0.01, Figure 2A). Expression of PEPCK-C was not significantly changed by the high-fat-diet in the adipose tissue of obese mice. After insulin treatment, the PEPCK-C expression was increased in the adipose tissue (P<0.05, Figure 2B). The effect of insulin treatment on the expression of those two factors was stronger in the early treatment group than that of late treatment group (P<0.05). Expression ofAQP7 was not significantly changed in the adipose tissue of obese mice. However, the expression was decreased by insulin in adipose tissue (P<0.05, Figure 2C).
FAS, PEPCK-C, AQP7 expression levels in adipose tissue of C57BL/6 mice. (Aa) Fatty acid synthase (FAS) expression levels in adipose tissue in C57BL/6 mice detected by Western blot. (Ab) Graphic representation of relative density of FAS protein levels in adipose tissue, which were normalized to those of β-actin. (Ba). Phosphoenolpyruvate carboxykinase (PEPCK-C) expression levels in adipose tissue in C57BL/6 mice detected by Western blot. (Bb) Graphic representation of relative density of PEPCK-C protein levels in adipose tissue, which were normalized to those of β-actin. (Ca) Aquaporin 7 (AQP7) expression levels in adipose tissue in C57BL/6 mice detected by Western blot. (Cb) Graphic representation of relative density of AQP7 protein levels in adipose tissue, which were normalized to those of β-actin.
Adiponectin, visfatin, and IL-6 expression
Expression of adiponectin protein was significantly decreased by high-fat-diet in the adipose tissue of obese mice (P<0.01). The expression was restored by insulin in both INS1 and INS2 groups (P<0.01) (Figure 3A). Expression of visfatin protein was increased in the adipose tissue of obese mice (P<0.01). The increase was not changed by insulin (Figure 3B). There was no significant difference in IL-6 protein levels between the lean (NC) and obese (FC) groups in the adipose tissue. However, insulin treatment significantly decreased IL-6 expression in the adipose tissue (P<0.01, INS1 vs FC; P<0.05, INS2 vs FC). This effect of insulin was stronger in the early insulin intervention group (P<0.05) (Figure 3C).
Adiponectin, visfatin, and IL-6 expression levels in adipose tissue of C57BL/6 mice. (Aa) Adiponectin expression levels in adipose tissue in C57BL/6 mice detected by Western blot. (Ab) Graphic representation of relative density of adiponectin protein levels in adipose tissue, which were normalized to those of β-actin. (Ba) Visfatin expression levels in adipose tissue in C57BL/6 mice detected by Western blot. (Bb) Graphic representation of relative density of visfatin protein levels in adipose tissue, which were normalized to those of β-actin. (Ca) Interleukin-6 (IL-6) expression levels in adipose tissue in C57BL/6 mice detected by Western blot. (Cb) Graphic representation of relative density of IL-6 protein levels in adipose tissue, which were normalized to those of β-actin.
Discussion
The metabolic and endocrine functions of adipose tissue are closely associated with the systemic insulin sensitivity. The primary function of adipose tissue is to store energy, mainly in the form of TG. If this function for adipocytes to synthesize and store TG is damaged, the spilled lipid could induce insulin resistance elsewhere. PEPCK-C and FAS are two key factors involved in the TG synthesis in adipose tissue. Our study found that 4-week insulin intervention in C57BL/6 mice increased the expression of PEPCK-C and FAS, indicating TG synthesis in adipose tissue may be increased in those mice. Inhibition of AQP7 expression by insulin may also contribute to TG synthesis as glycerol secretion is likely reduced. This change in adipose tissue may be responsible for reduction of TG accumulation in the liver and skeletal muscle. With insulin treatment, TG is likely transported from these two tissues to the adipose tissue, in which TG synthesis is enhanced by insulin. The triglyceride levels were significantly increased in liver, but only slightly elevated in muscle of obese mice in our study. This is consistent to a study11, in which the triglyceride concentration in liver was found to be more tightly related to insulin resistance. PEPCK-C is an enzyme involved in glucose and lipid metabolism. Our study found that insulin treatment, especially at early stage, could stimulate PEPCK-C expression in adipose tissue, while in other studies, insulin was demonstrated to inhibit PEPCK-C expression in liver tissue12, 13. The discrepancy may lie on the tissue-specific function and control of PEPCK-C. In liver tissue, PEPCK-C is involved in gluconeogenesis and is stimulated by glucocorticoid. While in adipose tissue, PEPCK-C is involved in glycerol synthesis and is inhibited by the same hormone-glucocorticoid. Another study also reported that insulin efficiently increased the storage of triglyceride in adipocytes by decreasing lipolysis and repressing energy expenditure14.
Endocrine function is another important function of adipose tissue. Our study also found that insulin treatment improved endocrine and immune functions of adipose tissue through regulating the expression of adiponectin and IL-6. Adiponectin, a 29-kDa adipocyte-derived hormone, is decreased with increased adiposity and has been linked to the insulin resistance15, 16, 17. In our present study, the protein expression of adiponectin was also found to be decreased by the high-fat-diet. With insulin treatment, adiponectin expression was significantly increased in the obese mice. Our result is consistent with observation in other study10, in which mRNA concentration encoding adiponectin was increased significantly by acute euglycaemic hyperinsulinaemia in the insulin-sensitive adipose tissue. Insulin has also been reported to increase adiponectin secretion from omental but not subcutaneous adipocyte in human18. These studies suggest that adiponectin is a sensitive target gene of insulin. The increased adiponectin expression may exert beneficial influence on systemic insulin sensitivity.
Visfatin is a cytokine that is highly expressed in visceral fat and whose blood levels correlate with obesity. It is still uncertain about the relationship between visfatin and insulin resistance, however, it has been suggested that serum visfatin increases with progressive beta cell deterioration19, 20. Our present study found that the expression of visfatin in adipose tissue was slightly increased in high-fat-diet induced obese mice, while insulin treatment seemed to exert no obvious effects.
IL-6 is an inflammatory cytokine secreted by adipocytes. Our study found that early insulin treatment significantly decreased IL-6 expression, which was inconsistent to a previous study, in which acute insulin infusion significantly stimulated the expression of IL-6, especially in insulin-resistant adipose tissue10. The discrepancies may be due to the different experimental procedures used (acute vs chronic).
In addition to these above effects by insulin, we also found that insulin improved glucose tolerance in obese mice, indicating that insulin could improve beta cell function, which was consistent to our clinical studies1, 2.
Taken together, our present study demonstrated that 4-week insulin treatment stimulated triglyceride synthesis and partly improved endocrine functions of adipose tissue. The mechanism is related to increased expression of PEPCK-C, FAS, adiponectin, and decreased expression of AQP7 and IL-6. TG content in liver and skeletal muscle was also reduced by insulin. These changes in fat, liver and skeletal muscle tissues may contribute to the mechanism of insulin action in the improvement of systemic insulin sensitivity.
Author contribution
Jian-ping WENG and Xiang CHEN designed and organized the research; Xiang CHEN, Qiu-qiong YU, yan-hua ZHU, Yan BI, Wei-ping SUN, Hua LIANG, Meng-ying CAI, and Xiao-ying HE performed research; Xiang CHEN wrote the paper.
References
Weng J, Li Y, Xu W, Shi L, Zhang Q, Zhu D, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet 2008; 371: 1753–60.
Li Y, Xu W, Liao Z, Yao B, Chen X, Huang Z, et al. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients is associated with improvement of beta-cell function. Diabetes Care 2004; 27: 2597–602.
Ryan EA, Imes S, Wallace C . Short-term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care 2004; 27: 1028–32.
Matthaei S, Stumvoll M, Kellerer M, Haring HU . Pathophysiology and pharmacological treatment of insulin resistance. Endocr Rev 2000; 21: 585–618.
Tordjman J, Chauvet G, Quette J, Beale EG, Forest C, Antoine B . Thiazolidinediones block fatty acid release by inducing glycero-neogenesis in fat cells. J Biol Chem 2003; 278: 18785–90.
Bogacka I, Xie H, Bray GA, Smith SR . The effect of pioglitazone on peroxisome proliferator-activated receptor-gamma target genes related to lipid storage in vivo. Diabetes Care 2004; 27: 1660–7.
Kishida K, Kuriyama H, Funahashi T, Shimomura I, Kihara S, Ouchi N, et al. Aquaporin adipose, a putative glycerol channel in adipocytes. J Biol Chem 2000; 275: 20896–902.
Hibuse T, Maeda N, Funahashi T, Yamamoto K, Nagasawa A, Mizunoya W, et al. Aquaporin 7 deficiency is associated with development of obesity through activation of adipose glycerol kinase. Proc Natl Acad Sci USA 2005; 102: 10993–8.
Waki H, Tontonoz P . Endocrine functions of adipose tissue. Annu Rev Pathol 2007; 2: 31–56.
Westerbacka J, Corner A, Kannisto K, Kolak M, Makkonen J, Korsheninnikova E, et al. Acute in vivo effects of insulin on gene expression in adipose tissue in insulin-resistant and insulin-sensitive subjects. Diabetologia 2006; 49: 132–40.
Anderwald C, Bernroider E, Krssak M, Stingl H, Brehm A, Bischof MG, et al. Effects of insulin treatment in type 2 diabetic patients on intracellular lipid content in liver and skeletal muscle. Diabetes 2002; 51: 3025–32.
O'Brien RM, Granner DK . Regulation of gene expression by insulin. Physiol Rev 1996; 76: 1109–61.
Hall RK, Yamasaki T, Kucera T, Waltner-Law M, O'Brien R, Granner DK . Regulation of phosphoenolpyruvate carboxykinase and insulin-like growth factor-binding protein-1 gene expression by insulin. The role of winged helix/forkhead proteins. J Biol Chem 2000; 275: 30169–75.
Nishino N, Tamori Y, Kasuga M . Insulin efficiently stores triglycerides in adipocytes by inhibiting lipolysis and repressing PGC-1alpha induction. Kobe J Med Sci 2007; 53: 99–106.
Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001; 7: 941–6.
Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G . Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes 2003; 52: 1779–85.
Yamamoto Y, Hirose H, Saito I, Tomita M, Taniyama M, Matsubara K, et al. Correlation of the adipocyte-derived protein adiponectin with insulin resistance index and serum high-density lipoprotein-cholesterol, independent of body mass index, in the Japanese population. Clin Sci (Lond) 2002; 103: 137–42.
Motoshima H, Wu X, Sinha MK, Hardy VE, Rosato EL, Barbot DJ, et al. Differential regulation of adiponectin secretion from cultured human omental and subcutaneous adipocytes: effects of insulin and rosiglitazone. J Clin Endocrinol Metab 2002; 87: 5662–7.
Lopez-Bermejo A, Chico-Julia B, Fernandez-Balsells M, Recasens M, Esteve E, Casamitjana R, et al. Serum visfatin increases with progressive beta-cell deterioration. Diabetes 2006; 55: 2871–5.
Chen MP, Chung FM, Chang DM, Tsai JC, Huang HF, Shin SJ, et al. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2006; 91: 295–9.
Acknowledgements
This work was supported by the National Basic Research Program (Profile of 973 Program, No 2006CB503902).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, X., Yu, Qq., Zhu, Yh. et al. Insulin therapy stimulates lipid synthesis and improves endocrine functions of adipocytes in dietary obese C57BL/6 mice. Acta Pharmacol Sin 31, 341–346 (2010). https://doi.org/10.1038/aps.2010.17
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2010.17
Keywords
This article is cited by
-
Zircon U–Pb age and geochemical constraints on the origin and tectonic implication of the Tuotuohe Cenozoic alkaline magmatism in Qinghai–Tibet Plateau
Acta Geochimica (2020)
-
Revisiting the metabolic syndrome: the emerging role of aquaglyceroporins
Cellular and Molecular Life Sciences (2018)
-
Suppression of Rho-kinase 1 is responsible for insulin regulation of the AMPK/SREBP-1c pathway in skeletal muscle cells exposed to palmitate
Acta Diabetologica (2017)
-
Anatomy of composition and nature of plate convergence: Insights for alternative thoughts for terminal India-Eurasia collision
Science China Earth Sciences (2017)