Abstract
Aim:
To investigate the effects of angiotensin receptor blocker (ARB) telmisartan on the expression and distribution of protein kinase C (PKC)-α in the kidneys of diabetic mice.
Methods:
Diabetic mice were induced with streptozotocin and a group of them were randomly selected for treatment with telmisartan. After 6 weeks, the expression and localization of PKC-α in the renal cortex, and the outer and inner medulla were assessed by immunohistochemistry and semiquantitative Western blotting. In addition, expressions of PKC-α, transforming growth factor-β1 (TGF-β1), and vascular endothelial growth factor (VEGF) in glomeruli were measured by semiquantitative immunohistochemistry.
Results:
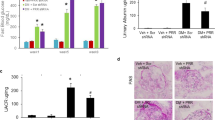
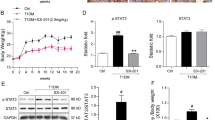
Diabetic and normal mice showed similar distributions of PKC-α in the kidneys. The expression of PKC-α was found in glomeruli, epithelial cells of proximal tubules, and medullary-collecting duct, while not in the medullary and cortical thick ascending limb, and was different in the epithelial cells of proximal tubules of diabetic nephropathy (DN) mice, PKC-α was mostly translocated from the basement membrane to the apical membrane, whereas it was largely translocated from the apical membrane to the basement membrane in epithelial cells of the inner medullary-collecting duct. Western blotting detected increased expression of PKC-α in the renal cortex and outer medulla, but not in the inner medulla of DN mice. Enhanced expressions of PKC-α, TGF-β1, and VEGF were shown in the glomeruli of DN mice, where PKC-α exhibited a correlation to VEGF, but no correlation to TGF-β1. ARB telmisartan attenuated alterations of PKC-α as mentioned earlier in the DN mice.
Conclusion:
Our findings suggest that PKC-α may play a role in the pathogenesis of DN, and that the nephroprotective effects of ARB telmisartan may be partly associated with its influence on PKC-α.
Similar content being viewed by others
Article PDF
References
Brownlee M . Biochemistry and molecular cell biology of diabetic complications. Nature, 2001; 414: 813–20.
Haller H . Postprandial glucose and vascular disease. Diabet Med, 1997; 14: S50–6.
Meier M, King GL . Protein kinase C activation and its pharmacological inhibition in vascular disease. Vasc Med, 2000; 5: 173–85.
Kang N, Alexander G, Park JK, Maasch C, Buchwalow I, Luft FC, et al. Differential expression of protein kinase C isoforms in streptozotocin-induced diabetic rats. Kidney Int, 1999; 56: 1737–50.
Thallas-Bonke V, Lindschau C, Rizkalla B, Bach LA, Boner G, Meier M, et al. Attenuation of extracellular matrix accumulation in diabetic nephropathy by the advanced glycation end product cross-link breaker ALT-711 via a protein kinase C-alpha-dependent pathway. Diabetes, 2004; 53: 2921–30.
Menne J, Park JK, Boehne M, Elger M, Lindschau C, Kirsch T, et al. Diminished loss of proteoglycans and lack of albuminuria in protein kinase C-α-deficient diabetic mice. Diabetes, 2004; 53: 2101–9.
Yao L, Huang DY, Pfaff IL, Nie X, Leitges M, Vallon V . Evidence for a role of protein kinase C alpha in urine concentration. Am J Physiol Renal Physiol, 2004; 287: F299–304.
Chen S, Hong SW, Iglesias-de la Cruz MC, Isono M, Casaretto A, Ziyadeh FN . The key role of the transforming growth factor-β system in the pathogenesis of diabetic nephropathy. Ren Fail, 2001; 23: 471–81.
Wolf G, Chen S, Ziyadeh FN . From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes, 2005; 54: 1626–34.
Chen S, Jim B, Ziyadeh FN . Diabetic nephropathy and transforming growth factor-beta: transforming our view of glomerulosclerosis and fibrosis build-up. Semin Nephrol, 2003; 23: 532–43.
De Vriese AS, Tilton RG, Elger M, Stephan CC, Kriz W, Lameire NH . Antibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetes. J Am Soc Nephrol, 2001; 12: 993–1000.
Flyvbjerg A, Dagnaes-Hansen F, De Vriese AS, Schrijvers BF, Tilton RG, Rasch R . Amelioration of long-term renal changes in obese type 2 diabetic mice by a neutralizing vascular endothelial growth factor antibody. Diabetes, 2002; 51: 3090–4.
Schrijvers BF, Flyvbjerg A, De Vriese AS . The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int, 2004; 65: 2003–17.
Cha DR, Kim NH, Yoon JW, Jo SK, Cho WY, Kim HK, et al. Role of vascular endothelial growth factor in diabetic nephropathy. Kidney Int Suppl, 2000; 77: S104–12.
Williams B, Gallacher B, Patel H, Orme C . Glucose-induced protein kinase C activation regulates vascular permeability factor mRNA expression and peptide production by human vascular smooth muscle cells in vitro. Diabetes 1997; 46: 1497–503.
Hoshi S, Nomoto K, Kuromitsu J, Tomari S, Nagata M . High glucose induced VEGF expression via PKC and ERK in glomerular podocytes. Biochem Biophys Res Commun, 2002; 290: 177–84.
Lindschau C, Quass P, Menne J, Guler F, Fiebeler A, Leitges M, et al. Glucose-induced TGF-β1 and TGF-β receptor-1 expression in vascular smooth muscle cells is mediated by protein kinase C-α. Hypertension, 2003; 42: 335–41.
Niimi Y, Mochida S, Matsui A, Inao M, Fujiwara K . PKC- and MAPK-independent upregulation of VEGF receptor expressions in human umbilical venous endothelial cells following VEGF stimulation. Hepatol Res, 2001; 21: 261–7.
Wolf G . New insights into the pathophysiology of diabetic nephropathy: from haemodynamics to molecular pathology. Eur J Clin Invest, 2004; 34: 785–96.
Makino H, Haneda M, Babazono T, Moriya T, Ito S, Iwamoto Y, et al. The telmisartan renoprotective study from incipient nephropathy to overt nephropathy-rationale, study design, treatment plan and baseline characteristics of the incipient to overt: angiotensin II receptor blocker, telmisartan, Investigation on Type 2 Diabetic Nephropathy (INNOVATION) Study. J Int Med Res, 2005; 33: 677–86.
Wienen W, Richard S, Champeroux P, Audeval-Gerard C . Comparative antihypertensive and renoprotective effects of telmisartan and lisinopril after long-term treatment in hypertensive diabetic rats. J Renin Angiotensin Aldosterone Syst, 2001; 2: 31–6.
Redling S, Pfaff IL, Leitges M, Vallon V . Immunolocalization of protein kinase C isoenzymes α, βI, βII, δ, and ζ in mouse kidney. Am J Physiol Renal Physiol, 2004; 287: 289–98.
Pfaff IL, Wagner HJ, Vallon V . Immunolocalization of protein kinase C isoenzymes α, βI and βII in rat kidney. J Am Soc Nephrol, 1999; 10: 1861–73.
Nishizuka Y . Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science, 1992; 258: 607–14.
Karim Z, Defontaine N, Paillard M, Poggioli J . Protein kinase C isoforms in rat kidney proximal tubule: acute effect of angiotensin II. Am J Physiol Cell Physiol, 1995; 269: 134–40.
Khundmiri SJ, Dean WL, McLeish KR, Lederer ED . Parathyroid hormone-mediated regulation of Na+-K+-ATPase requires ERK-dependent translocation of protein kinase Calpha. J Biol Chem, 2005; 280: 8705–13.
Liang M, Knox FG . Nitric oxide activates PKC-alpha and inhibits Na+-K+-ATPase in opossum kidney cells. Am J Physiol, 1999; 277: F859–65.
Bagrov YY, Manusova NB, Egorova IA, Fedorova OV, Bagrov AY . Endogenous digitalis-like ligands and Na/K-ATPase inhibition in experimental diabetes mellitus. Front Biosci, 2005; 10: 2257–62.
Hryciw DH, Pollock CA, Poronnik P . PKC-alpha-mediated remodeling of the actin cytoskeleton is involved in constitutive albumin uptake by proximal tubule cells. Am J Physiol Renal Physiol, 2005; 288: F1227–35.
Malhotra A, Kang BP, Cheung S, Opawumi D, Meggs LG . Angiotensin II promotes glucose-induced activation of cardiac protein kinase C isozymes and phosphorylation of troponin I. Diabetes, 2001; 50: 1918–26.
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by funds from Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (No 2005-21).
Rights and permissions
About this article
Cite this article
Yao, Lj., Wang, Jq., Zhao, H. et al. Effect of telmisartan on expression of protein kinase C-α in kidneys of diabetic mice. Acta Pharmacol Sin 28, 829–838 (2007). https://doi.org/10.1111/j.1745-7254.2007.00541.x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1111/j.1745-7254.2007.00541.x
Keywords
This article is cited by
-
Allisartan isoproxil reduces mortality of stroke-prone rats and protects against cerebrovascular, cardiac, and aortic damage
Acta Pharmacologica Sinica (2021)
-
Compounds from Lotus corniculatus
Chemistry of Natural Compounds (2019)
-
Molecular modeling studies of repandusinic acid as potent small molecule for hepatitis B virus through molecular docking and ADME analysis
Quantitative Biology (2019)