Abstract
Immunization with inactivated autoreactive T cells may induce idiotype anti-idiotypic reactions to deplete autoreactive T cells, which are involved in autoimmune diseases. However, it is unknown whether attenuated activated healthy autologous T-cell immunization could increase anti-tumor immune responses. To this end, C57Bl/6 mice were immunized with attenuated activated autologous T cells. The splenocytes from immunized mice showed a higher proliferative ability than that from naive mice. The special phenotype analysis showed that there were more CD8+ T cells and CD62L+ T cells in immunized mice after 24 h of culture with 10% fetal calf serum complete medium in vitro (P<0.01). These results demonstrated that this immunization may activate T cells in vivo. Furthermore, the splenocytes from immunized mice revealed resistance to activation-induced cell death (AICD) in vitro. To further study the relative genes that are responsible for the higher proliferation and resistance to AICD, the expression of Fas/Fas ligand (FasL) and GADD45b was measured by real-time PCR. The results indicated that GADD45β transcription was higher in the splenocytes from immunized mice than that in the naive mice. In addition, the Fas expression showed a parallel higher, but FasL did not change obviously. To investigate the biologic functions induced by immunization in vivo, a tumor model was established by EL-4 tumor cell inoculation in C57/Bl mice. Mice receiving autologous T-cell immunization had significantly inhibited tumor growth in vivo (P<0.01). This study implicated that immunization with attenuated activated autologous T cells enhances anti-tumor immune responses that participate in tumor growth inhibition.
Similar content being viewed by others
Introduction
It is well known that immune system has a surveillance function that mediates tumor growth inhibition in the body 1, 2. But during the development of cancer, tumor cells escape from immune surveillance due to limited effective anti-tumor immune responses in vivo 3, 4. Even though there are specific tumor antigens on the tumor cells that may induce anti-tumor immune responses and make tumor cells vanish occasionally, most malignant tumor cells grow in vivo without significant rejection by immune system 5. There are numerous factors involved in the survival of malignant tumor cells. For example, malignant tumor cells induce immune tolerance by different mechanisms 6, 7. In patients with malignancy, immune responses and reactivity to stimulators are significantly lower than that in healthy individuals 8. In addition, malignant tumor cells do not trigger specific immune responses; this is mediated at least in part by the secretion of inhibitory cytokines such as TGF-β and IL-10 9. Therefore, enhancing anti-tumor immune responses is a challenging task in designing and developing novel anti-tumor therapies 9, 10. Recently, many clinical trials have been developed for malignant tumor treatment, mainly by inducing anti-tumor-specific immune responses in the patients 10, 11. For example, dendritic cell (DC) vaccines are the most commonly used biotherapy for tumor immunotherapy 12. There are numerous DC vaccines that have been designed with gene modification or tumor antigen loading, which may deliver more tumor antigens and trigger stronger tumor rejection in vivo 13, 14, 15. Unfortunately, the DC vaccine strategy has limited utilization in clinic due to complications and high costs during preparation of this vaccine.
T cells play an important role in anti-tumor immune responses. T cells mediate cytotoxicity and immune regulation and maintain the immune system homeostasis 16, 17. Activated cytotoxic T lymphocytes (CTL) can lyse tumor cells directly, either by releasing granzyme B or by inducing apoptosis through Fas-Fas ligand (FasL) pathway 18, 19, 20. In addition, CD4+T cells can secrete cytokines that lead to immune rejection of tumor cells 21, 22, 23. However, the activity of T cells is down-regulated in patients with malignant tumors. For example, the tumor patients showed lower reactivity to mitogen or antigen stimulation in vitro 24. Therefore, it is critical to enhance anti-tumor T-cell functions in an effective immunotherapeutic design for tumor treatment 25. Accumulating evidence has demonstrated that tumor regression was induced in cancer patients treated with IL-2 plus IFN-γ 26, 27. This supports the idea that it is very important to enhance anti-tumor T-cell responses in the tumor immunotherapy 18, 19, 20, 21, 22, 23.
Administration of irradiated, activated autoimmune T cells to syngeneic animals or human patients may induce anti-idiotypic T-cell responses that lead to inhibition of in vivo autoimmune T-cell responses 28, 29. However, it is largely unexplored in tumor models with healthy inactivated autologous T-cell inoculation. Does it induce inhibition of T cell response in vivo? In this study, we demonstrate that inoculation with normal attenuated activated autologous T cells induced T-cell activation in vivo and stronger proliferation in vitro. Furthermore, this immunization enhanced anti-tumor immune responses and led to tumor growth arrest in vivo.
Materials and Methods
Mice
Female C57/Bl/6 mice were purchased from the Shanghai Laboratory Animal Center, Chinese Academy of Sciences. Mice were maintained under pathogen-free conditions and used between ages of 4 and 8 weeks.
Autologous activated T-cell preparation
Splenocytes (3×106/ml) were purified and stimulated with 2 μg/ml ConA (Vector Laboratories, Burlingama, CA, USA) in complete DMEM (DMEM with 10% fetal calf serum (FCS), 10 μM HEPES, 50 μM β-mercaptoethanol, 2 mM L-glutamine and 50 IU/ml penicillin-streptomycin) in 25 ml flasks for 72 h. The cells were collected and washed three times with PBS (pH 7.4). T cells were then purified using Dynabeads coated with specific antibodies by negative selection (Dynal Biotech, Oslo, Norway). The purity of T cells obtained from the culture was greater than 92%. Purified T cells were irradiated at 3000 rad with Irradiator as autologous activated healthy T cells for immunization as described below.
Optimal condition assay with irradiated autologous T-cell immunization
Three different T cell numbers, 1×106 (lower dose), 5×106 (middle dose) and 10×106 (high dose), were performed for immunization. Purified T cells were irradiated at 3000 rad and injected into mice in 100 μl PBS intraperitoneally with another 100 μl in PBS subcutaneously. The mice received two more similar immunizations every 5 days. As controls, 200 μl of the PBS were injected into the mice in the same way. Five days after the third immunization, the mice were killed and splenocytes were collected.
Proliferation assay
In brief, freshly isolated splenocytes (2×105 per well) were cultured in a flat-bottomed plate in triplicate in complete DMEM with or without ConA (2 μg/ml) at 37 °C in 5% CO2 for 72 h. Cells were pulsed with 1 μCi [3H]thymidine during the last 16-18 h of culture prior to harvest. [3H]thymidine incorporation was measured as c.p.m. using a beta-plate counter. The ability of T-cell proliferation was determined by [3H]thymidine incorporation. Stimulation index was calculated as (c.p.m. of T cells from immunization mice/that from naive mice) for mitogen response ability evaluation.
Cell surface molecule analysis by flow cytometry
For T-cell activation and assay of T-cell subsets, the T cells were incubated in 10% FCS complete medium for 24 h in vitro. The cells were collected and washed twice with HBSS buffer, 1×106 of the cells were stained with special goat anti-mouse CD3, CD4, CD8 and CD62L antibodies and detected by flow cytometry. For Fas and FasL analysis, the cells were cultured and collected at 24, 48, 72, 96 and 120 h, respectively. After the cells were washed twice with HBSS buffer, 1×106 of the cells were stained with special goat anti-mouse Fas and FasL and detected with flow cytometry. Briefly, cells were resuspended in PBS containing 1% bovine serum albumin (BSA) (Irvine) and 0.1% sodium azide (Sigma-Aldrich) and incubated with FITC- or PE-conjugated antibodies or isotype control antibodies at the recommended dilutions for 30 min on ice. Antibodies to Fas and FasL were obtained from BD Bioscience. Samples were analyzed using a FACS Calibur™ (Becton Dickinson, San Jose, CA, USA).
Apoptosis assay
The apoptotic cells were determined by FITC-labeled annexin V and propidium iodide (PI) staining and detected by flow cytometry. As the profile of apoptosis in T-cell subsets, the splenic cells were labeled by annexin V, PI and anti-CD4 or -CD8 antibodies (conjugated to Cy5) simultaneously. The cells from both naive and immunized mice were cultured in 10% FCS complete medium and apoptotic cells were detected at 48 h. The population of apoptotic cells was identified as annexin V+/PI-. The percentage of apoptotic cells was calculated according to total annexin V+/PI- divided by total cells.
mRNA expression detection by real-time PCR
The cells were cultured and collected at 24, 48, 72 and 96 h, respectively. After the cells were washed twice with HBSS buffer, total RNA was extracted from 1×106 of the cell pellets using RNeasy Mini Kit (Qiagen, Hilden, Germany). Genomic DNA was removed from total RNA prior to cDNA synthesis using the RNase-free DNase set for DNase digestion during RNA purification (Qiagen). RNA was stored at −80 °C. First-strand cDNA synthesis was performed for each RNA sample using Sensiscript RT Kit (Qiagen). Random hexamers (BioAsia Biotechnology, Shanghai, China) were used to prime cDNA synthesis. mRNA expression of Fas, FasL and GADD45β was determined by real-time PCR using SYBR Green master mix (Applied Biosystems, Foster City, CA, USA). Thermocycler conditions comprised an initial holding at 50 °C for 2 min, then 95 °C for 10 min, which was followed by a two-step PCR program consisting of 95 °C for 15 s, and 60 °C for 60 s for 40 cycles. Data were collected and quantitatively analyzed on an ABI Prism 7900 sequence detection system (Applied Biosystems). The mouse β-actin gene was used as an endogenous control to normalize for differences in the amount of total RNA in each sample. All quantities were expressed as number of folds relative to the expression of β-actin. PCR primer pairs were as follows: mouse Fas, 5′-GCA GAA AGT CCA GCT GCT CC and 3′-TTC TGC GAC ATT CGG CTT TT; FasL, 5′-CAC AAC CAC TCC CAC TGC C and 3′-CAG AGC CAC CAG AAC CAT GA; mouse GADD45β, 5′-CCT GGC AGA AAT CGC TAT GAA and 3′-TGG AGT AGT TTT GAG TGT GGA AGG; and mouse β-actin, 5′-TGT CCA CCT TCC AGC AGA TGT-3′ and 5′-AGC TCA GTA ACA GTC CGC CTA GA-3′.
Tumor growth inhibition assay
Mouse lymphoblastoma cell line EL-4 was used in this study. C57Bl/6 mice were divided into two groups. Control group: The mice were inoculated 1×106 of EL-4 tumor cells subcutaneously without immunization. Immunization group: C57 mice were immunized three times before inoculation with 0.5×106 EL-4 tumor cells. The tumor volume was measured every day. In this group, attenuated activated T cells were injected every week until the mice of control group were evaluated as dead according to the tumor size as described in pervious papers 30, 31. At the end of experiment, the mice were killed.
NK cytotoxicity assay
YAC-1 cells, a mouse myeloid leukemia cell line, were sub-cultured in RPMI-1640 complete cell culture medium supplemented with 10% FCS. The cell suspension was washed three times and re-suspended in RPMI-1640 as the target cells in all experiments. Single splenocytes were collected from naive and immunized mice as effect cells. The cytotoxic activity of NK was evaluated using the lactate dehydrogenase (LDH) release assays according to the manufacturer's protocol (Promega, WI, USA). Briefly, 5×104 target cells in assay buffer were added to each well of a 96 round-bottomed culture plate. Then, various numbers of effector cells in triplicate were added to test 4 effector:target cell ratios of 40:1, 20:1, 10:1 and 5:1 in the study. Four hours after incubation at 37 °C in 5% CO2, 100 μl of supernatant was transferred to the corresponding wells of a flat-bottomed microtiter plates. Subsequently, 100 ml of lactic acid dehydrogenase mixture (5.4×10−2 M(+) lactate, 6.6×10−4 M 2-p-iodophenyl-3-p-nitrophenyl tetrazolium chloride, 2.8×10−4 M phenazine methosulfate and 1.3× 10−3 M oxidized nicotinamide adenine dinucleotide in 0.2 M Tris-HCl (pH 8.2)) was added. After 5 min incubation at 37 °C, the absorbance at 490 nm was examined on an automated optical microplate reader (Spectra MAX 250, US). The cytotoxicity was calculated according to the following formula: % cytotoxity = (Experimental-effector spontaneous-target spontaneous)/(target maximum-target spontaneous LDH release) × 100.
CTL cytotoxicity assay
In this assay, EL-4 mouse thymic lymphoblastoma cells were sub-cultured in 10% FCS RPMI-1640 complete medium. A cell suspension was washed three times with PBS and re-suspended in RPMI-1640 as the target cells in all experiments. CD8+ T cells were purified from freshly isolated splenocyte with negative selection by Dynol beads. The cytotoxic activity of CTL was evaluated using LDH release assays described as in NK assay above. A serial E:T ratios of 10:1, 5:1, 2.5:1 and 1.25:1 were used in this assay. The reaction and detection conditions are the same as in NK assay described above.
Cytokine measurements by enzyme-linked immunosorbent assay (ELISA)
Sera were collected from both immunized mice and naive mice before they were killed. The serum samples were tested in duplicate for the production of IL-12, IL-10, IL-4 and TNF-α using ELISA kits, according to the manufacturer's instructions (BD Pharmingen, San Diego, CA, USA). Briefly, microtiter plates were coated overnight at 4 °C with mouse monoclonal anti-human TNF-α, IFN-γ, IL-12 and IL-10 antibodies, respectively (capturing antibody). Wells were then blocked at room temperature for 2 h with PBS containing 3% BSA (Sigma) and washed four times with PBS containing 0.02% Tween 20. Each sample and its control were added to adjacent wells and incubated overnight at 4 °C. After washing four times, appropriate biotinylated-detecting antibody (0.25 μg/ml) was added and incubated at RT for 2 h. Plates were washed four times and incubated for 30 min with avidin-conjugated horseradish peroxidase at 1:2000 dilution. As a substrate, 0.0123% tetramethylbenzidine/0.008% H2O2 in citrate buffer (pH 5.0) was used. The color development was stopped after 20 min at room temperature incubation using 2 N H2SO4. Optical densities were measured using an ELISA reader at 450 nm and the concentration of cytokine was calculated.
Statistical analysis
Differences in the proliferation and tumor volume between the groups were analyzed by the Mann-Whitney U-test. A Student's t-test was used to analyze the differences between the groups. One-way ANOVA was initially performed to determine whether an overall statistically significant change existed before using the two-tailed paired or unpaired Student's t-test. P-value of <0.05 was considered statistically significant.
Results
Spontaneous proliferation of T cells in vitro induced by autologous T-cell immunization
The immunized mice received 5×106 of attenuated autologous activated T cells in 100 μl PBS intraperitoneally with another 100 μl in PBS subcutaneously. After two more similar immunizations, the mice were killed and single splenocytes were isolated. To investigate whether autologous T-cell immunization can enhance T-cell responses, freshly isolated splenocytes from immunized C57 mice were cultured in 10% FCS complete DMEM with ConA (positive control) and without ConA. Cell proliferation was measured at 72 h after culture. The results showed that splenocytes from immunized mice had a high spontaneous proliferation in 10% FCS complete medium without any other stimulation. In contrast, splenocytes from PBS-treated mice showed no spontaneous proliferation in 10% FCS complete medium. There was a significant difference between immunized and naive mice (Figure 1, bar labeled with medium and double asterisks (**) on the top, P=0.0045). However, splenocytes from immunized or naive mice had a comparable level of proliferation in response to ConA stimulation, showing no significant difference (Figure 1, labeled by ConA, P=0.0839). This indicated that the splenocytes from immunized mice had almost same proliferation ability to ConA stimulation as that of from naive mice.
Spontaneous proliferation of T-cell in vitro induced by autologous T-cell immunization. Splenocytes from both naive (without immunization with attenuated activating autologous T cells, n=3) and immunized C57 mice (immunization with attenuated activated autologous T cells, n=3) were incubated in complete medium containing 10% FCS. ConA (2 μg/ml) was added to the culture as a positive control. The proliferation was measured by [3H]thymidine incorporation. The label “medium” represents that there was 10% of FCS in the medium only. The label “ConA” meant that there was ConA in medium with 10% FCS. The data shown here were from one representative of six experiments.
To evaluate whether the immunization induces anti-T-cell receptor (TCR) antibodies that could mediate T-cell proliferation in vitro, the sera from immunized mice were added into the splenic cells from immunized and naive mice, then subsequently cultured for 3 days in vitro. The proliferation was measured. The results displayed that the sera from immunized mice did not trigger proliferation of T cells from immunized mice. There was no proliferation found when the cells from both immunized and naive mice co-cultured with sera from either immunized mice or naive mice (data not shown). Moreover, we analyzed the cytotoxicity of T cells from immunized mice. The result demonstrated that there was no cytotoxicity observed when the CTL (effector cells) were incubated with CD4+ T cells (target cells) (data not shown). This suggested that the immunization protocol used in this study induced neither enough anti-TCR antibodies that engage TCR and activate T cells proliferation nor anti-TCR idiotype regulation.
T-cell expansion and activation induced by autologous T-cell immunization
To determine whether T-cell populations increased after immunization, the T-cell subsets were identified by flow cytometry after staining with specific antibodies. The results showed that the numbers of CD3+, CD4+ and CD8+ T cells from immunized mice were significantly higher than those from naive mice (P-values were 0.012, 0.005 and 0.002, respectively) (Figure 2A). This implicated that immunization with autologous T cell might stimulate T-cell population expansion in vivo. Furthermore, to address whether the increased T cells were activated by immunization, the anti-CD62L antibody staining assay was performed because CD62L usually is used as early activation marker of T cell. The results showed that there were over five times more CD62L-positive T cells from immunized mice than from naive mice. The counted cells between the two groups showed significant differences (Figure 2B, P<0.01). These results strongly suggested that immunization with irradiated autologous T cells increased T-cell population and induced T-cell activation simultaneously in vivo, which further led to T-cell spontaneous proliferation in vitro.
T-cell expansion and activation induced by autologous T-cell immunization. The splenic cells were incubated with FCS only for 24 h in vitro and 1×106 of the cells were stained with goat anti-mouse CD3, CD4, CD8 and CD62L antibodies and detected by flow cytometry. The open and black bars represent the cells isolated from naive and immunized mice, respectively, in both (A) and (B). (A) T-cell subset profile in both naive and immunized mice. (B) T-cell activation assay using anti-CD62L as activation marker.
Splenocytes from immunized mice are resistant to activation-induced cell death (AICD) in vitro
To address whether immunization increased T-cell-resisted AICD in vitro, the apoptotic cells were examined using annexin V plus PI staining and detected with flow cytometry after 48 h in culture. The results indicated that there were more annexin V+ and PI- cells in gated naive lymphocytes than that in gated immunized mice (Figure 3A). There were more apoptotic cells from naive mice than in immunized mice (P<0.05, Figure 3B). Furthermore, we labeled splenic cells with annexin V, PI and anti-CD4 or CD8 antibodies (labeled by Cy5) simultaneously to identify the apoptosis profile of T-cell subsets after 48 h culture. In the naive mice, both CD4+ and CD8+ T cells showed a similar enhanced rate of apoptosis. On the other hand, in the immunized mice, T-cell subsets showed much less apoptosis, especially CD4+ T cells (P<0.05, Figure 3C).
Splenocytes from immunized mice were resistant to AICD in vitro. The splenic apoptotic cells were examined using annexin V plus PI staining and detected with flow cytometry when the cells were cultured 48 h in vitro with 10% FCS complete medium. Only annexin V+/PI- cells were calculated as apoptotic cells at the checkpoint. For analysis of apoptotic T-cell subsets, the cells were labeled by annexin V, PI, and anti-CD4 or-CD8 antibodies (labeled by Cy5) simultaneously. (A) The dot plots of flow cytometry represent apoptotic cells in gated lymphocytes from naive (left) and immunized mice (right). (B) The percent of apoptotic cells after culturing 48 h in vitro with 10% FCS complete medium. (C) The apoptosis profile in T-cell subsets at the 48 h culture. The open bar represents the percent of apoptotic cells in naive mice. The black bar represents the percent of apoptotic splenic cells from immunized mice.
Because Fas and FasL are critical modulators of AICD, we examined the Fas and FasL kinetic expression in the T cells from both immunized and naive mice by real-time PCR. The results revealed that the Fas expression was increased with culture in vitro. The kinetic expression displayed that the Fas transcription started at 24 h and maintained higher expression until 96 h culturing. At every checkpoint, Fas expression in the immunized mice was higher than that in the naive mice. On the contrary, FasL expression was lower in immunized mice than that in naive mice. These results implicated that immunization with attenuated autologous T cells increased Fas molecule transcription but failed to increase FasL molecule expression in the T cells with culture in vitro (Figure 4A and 4B).
Timing analysis of Fas and FasL transcription in naive or immunized mice at different time points in vitro. Spleen cells from both naive (n=4) and immunized mice (n=4) were cultured in 10% FCS complete medium. At 0 h (freshly isolated cells), 24 h, 48 h, 72 h and 96 h culture, total RNA was extracted and mRNA level of Fas and FasL was analyzed using real-time PCR. Fas expression was normalized to endogenously expressed GAPDH in the same samples. Relative expression was calculated as the difference (ΔΔCT) between the ΔCT values of immunized mice and of the endogenous control (GAPDH). Relative expression of Fas/FasL gene was calculated and expressed as 2-ΔΔCT. (A) Fas expression in naive mice (open bar) and immunized mice (black bar). (B) FasL expression in naive mice (open bar) and immunized mice (black bar).
High expression of GADD45β in the T cells from immunized mice
As GADD45β is a potential inhibitor for AICD, the GADD45β expression in the T cells from immunized mice was also investigated by real-time PCR at different times. The result indicated that the mRNA level of GADD45β was higher in immunized mice than in naive mice at different time points (Figure 5). The GADD45β expression in the freshly isolated T cells was three times higher than that of naive mice. This indicated that autologous T-cell immunization triggered the gene transcription in vivo. In culture with 10% FCS complete medium, the mRNA of GADD45β increased and achieved a maximal induction at 16 h. After that, the transcription of mRNA of GADD45β decreased slowly. By contrast, in the naive mice, the mRNA level of GADD45β rose to an intermediate level and reached the highest level at 8 h. But after 8 h, the GADD45β level quickly decreased and went down to baseline at 48 h in vitro. This result demonstrated that GADD45β transcription was activated in vivo through immunization, which might account for splenocyte resistance to apoptosis in immunized mice (Figure 5).
Higher expression of Gadd45β in the T cells from immunized mice. T cells from immunized mice (n=4) were cultured in 10% of FCS complete medium. The expression of GADD45β was determined by real-time PCR and Western blot. Total RNA was extracted at 0 h (freshly isolated T cells), 2 h, 8 h, 16 h, 24 h and 48h for real-time PCR analysis. GADD45β expression was normalized to endogenously expressed GAPDH in the same samples. Relative expression was calculated as the difference (ΔΔCT) between the ΔCT values of immunized mice and of the endogenous control (GAPDH). Relative expression of GADD45β gene was calculated and expressed as 2-ΔΔCT.
Inhibition of tumor growth by autologous T-cell immunization in vivo
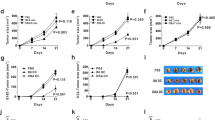
To test whether spontaneous cell proliferation mediated by autologous T-cell immunization leads to enhanced anti-tumor responses, the mouse lymphoblastoma cell line EL-4 was inoculated into immunized mice. Tumor growth was observed in both immunized and control (unimmunized) mice. As indicated in Figure 6A, tumor growth was slower after the final immunization (after 10 days of tumor cell injection). The tumor volume was significantly smaller than that of control group at 12 days of tumor inoculation (P=0.0441). Similarly, at the end of observation (14 days after tumor cell inoculation), the tumor volume in the immunization group was significantly smaller than that of the tumor in the control group (P=0.0160). This implicated that the tumor growth was antagonized in the immunized mice.
The immunization protection from tumor challenge. Animals were divided into two groups. Mice in the control group (n=5) received 1×106 of EL-4 tumor cell inoculation alone without attenuated activated autologous T-cell immunization. Immunization group: mice (n=5) were immunized three times every 5 days before 1×106 of EL-4 tumor challenge followed by another twice immunization after 5 and 10 days challenge, respectively. The tumor volume was measured every 2 days after tumor cell inoculation. When the tumor volume reached 2.0 cm3, the observation was ended. The mice were then killed and sera were obtained for cytokine and NK plus CTL detection. (A) Tumor volume with days after EL-4 tumor cell inoculation. The tumor volume in control group (○) compared with the tumor volume in immunized group (•). The results represented one of three experiments. (B) Cytokine profile in control group (open bar) and immunized group (black bar). (C) NK cytotoxicity in naive mice (open circle) and immunized mice (solid circle). (D) CTL cytotoxicity in naive mice (open circle) and in immunized mice (solid circle).
IL-12 is induced in immunized mice
Sera were collected from both immunized mice and naive mice before they were killed. The serum samples were tested in duplicate for the production of IL-12, IL-10, IL-4 and TNF-a using ELISA kits. In this assay, only IL-12 levels were increased in immunized mice compared to that from sera of control mice (P=0.0023) (Figure 6B). This revealed that immunization with autologous T cells might produce more IL-12 that may activate anti-tumor T-cell responses to mediate tumor growth inhibition in vivo.
NK and CTL activity increased in the immunized mice
Single splenocytes were collected from naive and immunized mice as effector cells. The cytotoxic activity of NK was evaluated using LDH release assays. The results indicated that both NK and CTL cytotoxicities were higher in immunized mice than in the naive mice. In NK assay, we used the splenic cells as effector cells. The cytotoxicity supplied by splenic cells from immunized mice showed that there was an obviously dose-dependent LDH release from higher E:T to lower E:T. But the NK lysis was not obviously observed in the naive mice (Figure 6C). In CTL cytotoxicity assay, samples from CD8+ T cells from immunized mice demonstrated higher OD value supported by more LDH release after the CTLs were incubated with EL-4 target cells. Similar to what was observed in the NK assay, the cytotoxicity showed an obviously dose-response relationship (Figure 6D). These data strongly implicated that immunization increased NK cytotoxicity. In the CTL cytotoxicity assay, the results displayed that the immunization induced anti-tumor capacity that may not be mediated by TCR recognition.
Discussion
It is well known that injection of irradiated, activated autoimmune T cells to syngeneic animals or human patients may induce anti-idiotypic T cells in vivo, which inhibited or deleted autoimmune T cells in some cases 32. For example, in multiple sclerosis studies, accumulated evidence supports the idea that autoimmune T cells can be modulated by regulatory T cells and are deleted from peripheral blood. This was seen with remission of MS symptoms. Clinical trials with T-cell vaccines have demonstrated that inoculation of patients with attenuated autologous T cells is safe 32, 33. In this study, we addressed for the first time that immunization with attenuated activated normal autologous T cells promotes tumor growth arrest as a consequence of T cells activated in vivo.
The most interesting phenomenon in our study was that freshly isolated splenocytes from immunized mice proliferated spontaneously. This proliferation was observed in only 10% FCS, but without any other stimulators. This means that the immunization triggered T cells in vivo. To eliminate the possibility that T-cell proliferation was mediated by anti-TCR antibodies induced by immunization with autologous T cells, we examined the ability of sera from immunized mice to induce proliferation by adding the immunized sera into T-cell cultures. The results showed that the sera from immunized mice did not induce proliferation of T cells from immunized mice, suggesting that the immunization procedure used in our study did not induce enough anti-TCR antibodies to initiate TCR engagement and continuously evoke T-cell proliferation. Moreover, the cytotoxicity assay demonstrated that there was no cytotoxicity observed when the CD8+ T cells met CD4+ T cells from the same immunized mice. This suggested that the mice immunized three times during 15 days did not induce enough anti-TCR antibodies and anti-idiotype CTL. We propose that the immunization in our study indeed promoted T-cell activation independent of the TCR pathway and induced T cells to be more susceptible to stimulation from any other exogenous protein or mitogen.
To further investigate whether the T-cell population responded to immunization, we analyzed the T cells expanded in vivo by detecting T-cell populations with flow cytometry. The results implicated that the CD3+ T-cell population was expanded after immunization in vivo. This suggested that the autologous T-cell immunization triggered T-cell expansion in vivo. Further, we studied T-cell activation by immunization. T cells cultured in 10% FCS complete medium were stained with anti-CD62L antibody and the positive cells were captured by flow cytometry. The results showed that the CD62L expression was significantly higher on T cells from immunized mice than on T cells from naive mice. This confirmed that immunization with irradiated autologous T cells increased T-cell populations and induced T-cell activation simultaneously in vivo, which further led to T-cell spontaneous proliferation in vitro.
AICD is well known in a normal immune reaction in which the lymphocytes become activated and proliferated under stimulation, and later underwent programmed cell death. This process plays a very important role in immune homeostasis 34, 35. There are many factors involved in the AICD. Fas and FasL are very important proteins in the AICD process 36, 37. Fas is a molecule expressed on activated T cells and serves as an activation marker for T cells. When the lymphocytes are stimulated with antigens, the Fas gene starts transcription and the Fas protein is expressed on the cell surface. Later, activated lymphocytes produce FasL and express it on the cell surface. FasL can bind the extracellular region of the Fas molecule and result in the activation of the Fas-coupled death domain in the cytoplasm. Once the FasL combines with Fas expressed either on the same or other cells, the downstream caspase cascades are triggered. This reaction leads to apoptosis that blocks further activation of lymphocytes 38.
To address the fate of T cells from immunized mice, we detected the apoptosis pattern of proliferated T cells from naive and immunized T cells. The results showed that there were fewer apoptotic cells from immunized mice than from naive mice. Among apoptotic cells, CD4+ T cells were less sensitive to apoptosis than CD8+ T cells in the immunized mice. But there was no difference in T-cell subset from naive mice. These data indicated that the cells from immunized mice not only exhibited stronger proliferation but also lived longer in vitro. These implicated that the cells from immunized mice were susceptible to external stimulation and were resistant to AICD triggered by stimulation.
To investigate the mechanism of higher ability of response and resistance to AICD mediated by immunization, the transcription of Fas and FasL was detected by real-time PCR every 24 h during the culture. The Fas mRNA level in the splenocytes from immunized mice was higher than that from naive mice, but FasL mRNA in immunized mice was lower than that in naive mice. At most time points described above, FasL was obviously lower in the cells from immunized mice than in those from naive mice. This means that immunization induced Fas expression in T cells, but that the FasL transcription was not upregulated simultaneously. It is well known that high Fas with low FasL will keep the activated T cells resistant to AICD 37, 38.
It is very interesting to ask which molecules or genes were involved in the resistance to AICD after immunization. The GADD (growth arrest and DNA damage-inducible) family consists of three genes (GADD45α, GADD45β and GADD45γ) that are evolutionarily well conserved; the molecular functions of their gene products are very similar when they are overexpressed in cell lines 39. However, these genes are regulated differently and as a consequence their in vivo functions are different. GADD45β is involved in cell cycle arrest, apoptosis, signal transduction and cell survival. In T cells, GADD45β was rapidly induced by TCR and inflammatory signals. Deficiency of GADD45â in CD4+ T cells impaired their responses to TCR stimulation by inflammatory cytokines 40, 41. Previous work have shown that GADD45β inhibits cell apoptosis by NF-κB downregulation through JNK signaling pathway in many tumor cells or cell lines 42, 43. Moreover, recent reports demonstrated that the GADD45β protects significantly against Fas-induced AICD in both T and B cells and maintains cell viability by NF-kB activation 44, 45.
As GADD45β is a potential inhibitor for AICD, we tested the timing of GADD45β expression. GADD45β transcription in the freshly isolated T cells from immunized C57 mice was higher than in T cells from naive mice. This meant that the immunization promoted GADD45β expression in vivo. In vitro experiments indicated that immunization resulted in the rapid increase of GADD45β mRNA in the presence of FCS and reached the highest level at 16 h, then subsided slowly. The level of GADD45β in immunized mice was higher than that in the naive mice at every examined time point. By contrast, in naive mice, the mRNA expression of GADD45β increased under FCS stimulation and reached highest level early and decreased more rapidly. These results implied that the GADD45β transcription was promoted in vivo and maintained longer by immunization. Consistent with the higher level of GADD45β, the T cells were protected against Fas-induced apoptosis. Whether and how GADD45β inhibited apoptosis mediated by Fas is under investigation. Akanksha et al. 44 reported that the TCR stimulation led to transient nuclear accumulation of the NF-kB, whichβ activated GADD45β expression and protected Fas-induced apoptosis subsequently. But in our study, immunization elicited T-cell activation and Fas expression by a mechanism distinct from TCR engagement, a different situation than that described by Akanksha et al. So, it is very interesting to determine the mechanism of GADD45β induction and its role in inhibition of AICD.
T cells play a very important role in tumor immunology. Both activated CTL and CD4+ T cells are effectors in the tumor rejection response in vivo. It is well known that CTL and NK cells can lyse tumor cells directly by either releasing granzyme B or by inducing apoptosis through the Fas-FasL or TNF-TNFR system 18, 19, 20. CD4+T cells can secrete cytokines and lead to tumor cell growth arrest 21, 22, 23. As discussed above, we found that T cells were activated and resistant to AICD after immunization. We tried to test whether T cells activated by immunization could inhibit tumor growth in vivo. In our study, the mice received three immunizations before EL-4 tumor cells were inoculated and received another two extra immunizations after the tumor cell injection. In these mice, the tumor growth was significantly inhibited compared to that of the naive mice. Both NK and CTL cytotoxicities increased after immunization. Meanwhile, the cytokine analysis showed that there was higher concentration of IL-12 in the immunized mice.
Recently, many reports demonstrated that IL-12 up-regulated GADD45β expression. On the other hand, GADD45β expression can induce IL-12 production. There is a positive feed loop between GADD45β expression and IL-12 production 40, 41, 43. Our results are consistent with the notion that GADD45β not only suppressed apoptosis induced by Fas but also up-regulated IL-12 production.
Our data implicated that immunization with normal attenuated activated autologous T cells induced tumor arrest in vivo. The inhibition of tumor growth was mediated by multiple factors, including T-cell activation, resistance to AICD, and higher levels of NK and CTL cytotoxicity. Our study provided the first evidence that immunization with healthy attenuated activated autologous T cells could enhance immune proliferation and induce resistance to AICD that may mediate tumor cell growth in the murine model. How immunization with T cells can lead to these effects and the underlying molecular mechanisms are under investigation.
References
Andersen MH, Pedersen LO, Becker JC, Straten PT . Identification of a cytotoxic T lymphocyte response to the apoptosis inhibitor protein surviving in cancer patients. Cancer Res 2001; 61:869–872.
Andersen MH, Pedersen LO, Capeller B, Brocker EB, Becker JC, thor Straten P . Spontaneous cytotoxic T cell responses against surviving-derived MHC class I-restricted T cell epitopes in situ as well as ex vivo in cancer patients. Cancer Res 2001; 61:5964–5968.
Cinatl Jr J, Scholz M, Doerr HW . Role of tumor cell immune escape mechanisms in cytomegalovirus-mediated oncomodulation. Med Res Rev 2005; 25:167–185.
Melchionda F, McKirdy MK, Medeiros F, Fry TJ, Mackall CL . Escape from immune surveillance does not result in tolerance to tumor-associated antigens. J Immunother 2004; 27:329–338.
Nishimura T, Iwakabe K, Sekimoto M, et al. Distinct role of antigen-specific T helper type I (Th1) and Th2 cells in tumor eradication in vivo. J Exp Med 1999; 190:617–627.
Fallarino F, Grohmann U, Bianchi R, Vacca C, Fioretti MC, Puccetti P . Th1 and Th2 cell clones to a poorly immunogenic tumor antigen initiate CD8+ T cell-dependent tumor eradication in vivo. J Immunol 2000; 165:5495–5501.
Yazawa T, Ito T, Kamma H, et al. Complicated mechanisms of class II transactivator transcription deficiency in small cell lung cancer and neuroblastoma. Am J Pathol 2002;161:291–300.
Acihini A, Maccalli C, Mortarini R, et al. Melanoma cells and normal melanocytes share antigens recognized by HLA-A2-restricted cytotoxic T cell clones from melanoma patients. J Exp Med 1993; 177:989–995.
Surman D, Dudley ME, Overwijk WW, Restifo NP . Cutting edge: CD4+ T cell control of CD8+ T cell reactivity to a model tumor antigen. J Immunol 2000; 164:562–565.
Dannull J, Su Z, Rizzieri D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest 2005; 115:3623–3633.
Liu M, Acres B, Balloul JM, et al. Gene-based vaccines and immunotherapeutics. Proc Natl Acad Sci 2004; 101(Suppl 2):14567–14571.
Escobar A, Lopez M, Serrano A, et al. Dendritic cell immunizations alone or combined with low doses of interleukin-2 induce specific immune responses in melanoma patients. Clin Exp Immunol 2005; 142:555–568.
Hanada T, Yoshida H, Kato S, et al. Suppressor of cytokine signaling-1 is essential for suppressing dendritic cell activation and systemic autoimmunity. Immunity 2003; 19:437–450.
Schnurr M, Galambos P, Scholz C, et al. Tumor cell lysate-pulsed human dendritic cells induce a T-cell response against pancreatic carcinoma cells: an in vitro model for the assessment of tumor vaccines. Cancer Res 2001; 61:6445–6450.
Shen L, Evel-Kabler K, Strube R, Chen SY . Silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity. Nat Biotechnol 2004; 22:1546–1553.
Graca L, Chen TC, Moine AL, et al. Dominant tolerance: activation thresholds for peripheral generation of regulatory T cells. Trends Immunol 2005; 26:130–135.
Peng SL . Fas (CD95)-related apoptosis and rheumatoid arthritis. Rheumatology 2006:45:26–30.
Pardo J, Bosque A, Brehm R, et al. Apoptotic pathways are selectively activated by granzyme A and/or granzyme B in CTL-mediated target cell lysis. J Cell Biol 2004; 167:457–468.
Chen SY, Yang A, Chen J, et al. Potent anti-tumor activities of a new class of tumor-specific killer cells. Nature 1997; 385:78–80.
Yang S, Haluska FG . Treatment of melanoma with 5-fluorouracil or dacarbazine in vitro sensitizes cells to antigen-specific CTL lysis through perforin/granzyme- and Fas-mediated pathways. J Immunol 2004;172:4599–4608.
Kalams SA, Walker BD . The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J Exp Med 1998; 188:2199–2204.
Huang Y, Obholzer N, Fayad R, Qiao L . Turning on/off tumor-specific CTL response during progressive tumor growth. J Immunol 2005; 175:3110–3116.
Campi G, Crosti M, Consogno G, et al. CD4+ T cells from healthy subjects and colon cancer patients recognize a carcinoembryonic antigen-specific immunodominant epitope. Cancer Res 2003; 63:8481–8488.
Hajnzic TF, Kastelan M, Lukac J, Hajnzic T . Immunocompetent cells and lymphocyte reactivity to mitogens in levamisole-treated brain tumor children. Pediatr Hematol Oncol 1999;16:335–340.
Tang J, Flomenberg P, Harshyne L, et al. Glioblastoma patients exhibit circulating tumor-specific CD8+ T cells. Clin Cancer Res 2005;11:5292–5299.
Aarnoudse CA, van den Doel PB, Heemskerk B, Schrier PI . Interleukin-2-induced, melanoma-specific T cells recognize CAMEL, an unexpected translation product of LAGE-1. Int J Cancer 1999; 82:442–451.
Liu SH, Zhang M, Zhang WG . Strategies of antigen-specific T-cell-based immunotherapy for cancer. Cancer Biother Radiopharm 2005; 20:491–501.
Hong J, Zang Y, Tejada-Simon M, et al. Reactivity and regulatory properties of human anti-idiotypic antibodies induced by T cell vaccination in patients with multiple sclerosis. J Immunol 2000; 165:6858–6864.
Shapira OM, Mor E, Reshef T, et al. Prolongation of survival of rat cardiac allografts by T cell vaccination. J Clin Invest 1993; 91:388–390.
Rakhmilevich AL, Timmins JG, Janssen K, et al. Gene gun-mediated IL-12 gene therapy induces antitumor effects in the absence of toxicity: a direct comparison with systemic IL-12 protein therapy. J Immunother 1999; 22:135–144.
Kanwar JR, Shen WP, Kanwar RK, Berg RW, Krissansen GW . Effects of survivin antagonists on growth of established tumors and B7–1 immunogene therapy. J Natl Cancer Inst 2001; 93:1541–1552.
Kumar V, Sercarz E, Zhang JW, Cohen I . T cell vaccination: from basics to the clinic. Trends Immunol 2001; 22:539–544
Kozovska ME, Hong J, Zang YCQ, et al. Interferon beta induces T-helper 2 immune deviation in MS. Neurology 1999; 53:1692–1697.
Lockshin RA, Zakeri Z . Apoptosis, autophagy and more. Int J Biochem Cell Biol 2004; 36:2405–2419.
Waring P, Mullbacher A . Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunol Cell Biol 1999; 77:312–317.
Greeneltch KM, Kelly-Welch AE, Shi Y, Keegan AD . Chronic morphine treatment promotes specific Th2 cytokine production by murine T cells in vitro via a Fas/Fas ligand-dependent mechanism. J Immunol 2005; 175:4999–5005.
Sun T, Zhou Y, Li H, et al. FASL -844C polymorphism is associated with increased activation-induced T cell death and risk of cervical cancer. J Exp Med 2005; 202:967–974.
Mallone R, Kochik SA, Reijonen H, et al. Functional avidity directs T-cell fate in autoreactive CD4+ T cells. Blood 2005; 106:2798–2805.
M Gupta, SK Gupta, AG Balliet, et al. Hematopoietic cells from Gadd45a- and Gadd45b-deficient mice are sensitized to genotoxic-stress-induced apoptosis. Oncogene 2005; 24:7170–7179.
Papa S, Zazzeroni F, Pham CG, et al. Linking JNK signaling to NF-kB: a key to survival. J Cell Sci 2004; 117:5197–5208.
Lu BF, Ferrandino AF, Flavell RA, et al. Gadd45b is important for perpetuating cognate and inflammatory signals in T cells. Nat Immunol 2004; 5:38–44.
Chi H, Lu BF, Takekawa M, Davis RJ, Flavell RA . GADD45b/GADD45c and MEKK4 comprise a genetic pathway mediating STAT4-independent IFNγ production in T cells. EMBO J 2004; 23:1576–1586.
Yang J, Zhu H, Murphy TL, et al. IL-18-stimulated GADD45 required in cytokine-induced, but not TCR-induced, IFN-γ production. Nat Immunol 2001; 2:157–164.
Mittal A, Papa S, Franzoso G, Sen RJ . NF-kB-dependent regulation of the timing of activation-induced cell death of T lymphocytes. J Immunol 2006; 176:2183–2189.
Azzzeroni F, Papa S, Algeciras-Schimnich A, et al. GADD45β– mediates the protective effects of CD40 costimulation against Fas-induced apoptosis. Blood 2003; 102:3270–3279.
Acknowledgements
This work was supported by Science and Technology Commission of Shanghai Municipality (Nos. 03DJ14009, 04DZ14902, 05ZR14055, 054319928), Shanghai Municipal Education (No. 05BZ26) and Shanghai Leading Academic Discipline Project (T0206), Translational Research Seed Fund of Institute of Health Sciences (No. XK2172).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, L., Du, F., Cao, Q. et al. Immunization with autologous T cells enhances in vivo anti-tumor immune responses accompanied by up-regulation of GADD45β. Cell Res 16, 702–712 (2006). https://doi.org/10.1038/sj.cr.7310083
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cr.7310083