ABSTRACT
Overexpression and activation of HER-2/neu (also known as c-erbB-2), a proto-oncogene, was found in about 30% of human breast cancers, promoting cancer growth and making cancer cells resistant to chemo- and radio-therapy. Wild-type p53 is crucial in regulating cell growth and apoptosis and is found to be mutated or deleted in 60-70% of human cancers. And some cancers with a wild-type p53 do not have normal p53 function, suggesting that it is implicated in a complex process regulated by many factors. In the present study, we showed that the overexpression of HER-2/neu could decrease the amount of wild-type p53 protein via activating PI3K pathway, as well as inducing MDM2 nuclear translocation in MCF7 human breast cancer cells. Blockage of PI3K pathway with its specific inhibitor LY294002 caused G1-S phase arrest, decreased cell growth rate and increased chemo- and radio-therapeutic sensitivity in MCF7 cells expressing wild-type p53. However, it did not increase the sensitivity to adriamycin in MDA-MB-453 breast cancer cells containing mutant p53. Our study indicates that blocking PI3K pathway activation mediated by HER-2/neu overexpression may be useful in the treatment of breast tumors with HER-2/neu overexpression and wild-type p53.
Similar content being viewed by others
INTRODUCTION
HER-2/neu (also known as c-erbB-2 ) gene encodes a 185-KD transmembrane growth receptor tyrosine kinase, which is partial homologous to other members of the epidermal growth factor receptor family 1. Amplification or overexpression of the HER-2/neu gene was found in up to 30% of primary breast and other human cancers, and it was often correlated with increased tumor invasion, poor prognosis and therapeutic resistance 2,3,4. However, the molecular mechanism for these phenomena has not been completely elucidated. It was reported that over-expression of HER-2/neu gene can activate the phospha-tidylinositol-3 kinase (PI3K)-Akt pathway without exogenous ligand stimulation 5, and PI3K-Akt pathway activation was also reported to be able to delay p53-mediated apoptosis 6. These findings suggest the over-expression of HER-2/neu may be involved in PI3K-Akt pathway activation and in turn p53-mediated apoptosis.
The product of wild-type p53 gene is a tumor suppressor that mediates growth arrest, senescence and apoptosis in response to several cellular stresses. p53 is frequently mutated or deleted in many types of tumors 7,8 and some others with a wild-type p53 gene do not possess normal p53 function, suggesting that it may be regulated by various oncogenic signals 9.
In the current study, we found that the over expression of HER-2/neu decreased the amount of wild-type p53 protein and induced MDM2 nuclear translocation by activating the PI3K-Akt pathway in MCF7 cells. Inhibition of PI3K-Akt pathway increased the amount of wild-type p53 protein, thus decreased cell growth rate and enhanced the sensitivity to chemotherapeutic drug adriamycin and γ-irradiation in HER-2/neu overexpressing cells. However, blocking PI3K pathway could not increase the sensitivity to adriamycin in human breast tumor cell line MDA-MB-453 that harbors mutant p53.
MATERIALS AND METHODS
Construction and reagents
pcDNA3.0-herw, which contains whole length of human HER-2/neu gene, was constructed by our laboratory. Tfx™-20 was purchased from Promega. Mouse anti-human HER-2/neu monoclonal antibody was prepared by our group as described previously 10. Mouse anti-human p53 monoclonal antibody (clone BP53-12) was purchased from Sigma. Rabbit anti-Akt, p-Akt polyclonal antibodies, goat anti-rabbit IgG-HRP, and PI3K pathway inhibitor LY294002, were purchased from Cell Signaling Technology. Rabbit anti-human MDM2 polyclonal antibody (H-221) was purchased from Santa Cruz. Goat anti-mouse IgG-HRP was obtained from Jackson. Adriamycin was provided by Zhejiang Haizheng Drug company. RPMI 1640, fetal bovine serum and Trizol kit were purchased from Invitrogen. Propidium iodide (PI) and tetrazolium-containing dye solution (MTT) were purchased from Merck. CelLytic™ Nuclear Protein Extraction Kit was kindly gifted by Dr. Chen Yang. BCA protein assay kit was purchased from Pierce Biotechnology, Inc. LY294002 was stored in DMSO and added to the medium to a final concentration of 25 μM. DMSO served as vehicle.
Cell culture
MCF7 and MDA-MB-453 human breast tumor cell lines were purchased from Type Culture Collection of Chinese Academy of Sciences. Cells were grown in RPMI 1640 medium supplemented with 10% heat inactivated fetal bovine serum. MCF7-neu3 (MCF7 cells transfected with pcDNA3.0-herw) and MCF7-pc3.0 (MCF7 cells transfected with pcDNA3.0) cells were maintained in the above medium containing 800 μg/ml G418. All cell lines were cultured at 37ºC under 5% CO2.
Plasmid transfection
Transfection was performed on 80% confluent MCF7 cells according to the manufacturer's instruction of Tfx™-20. Transfection mixture (per well in 6-well culture plates) was consisted of 4.5 μl Tfx™-20, 1.5 μg ultra-pure plasmid pcDNA3.0-herw and 900 μl optimum reduced serum free medium. Cells were washed once with optimum reduced serum free medium, then the transfection mixture was added. 8 h later, cell medium was replaced with fresh completed medium without antibiotics. After cultured for additional 48 h, cells were selected in medium containing G418 (800 μg/ml) for 20 d. Clones were in turn transferred to 24-well plates, 6-well plates and finally flasks.
Protein extraction and Western blot analysis
Cells were collected and lysed in ice-cold lysis buffer (50 mM NaCl, 0.01 M Tris-Cl pH8.0, 5 μM EDTA pH 8.0, 0.5% NP-40, 1 mM phenylmethysulfonyl fluoride, 1 μg/ml Aprotinin, 1 μg/ml Leupeptin). After incubation for 20 min on ice, cellular debris was removed by centrifugation at 10,000 g for 5 min, and then supernatants were transferred to new tubes. Nuclear protein was extracted according to the instruction of CelLytic™ Nuclear™ Extraction Kit. Proteins were quantified according to the instruction of BCA protein assay kit. Equal amounts of protein (30 μg per lane except for the detection of p53, for which 100 μg per lane was used) were seperated in SDS-polyacrylamide gels (6%-10%) and transferred to polyvinylidene difluoride membrane. After blocked with 5% BSA in Tris-buffered saline-Tween 20 for 1 h at room temperature, the membranes were incubated with the specific primary antibodies. Subsequently, membranes were washed and incubated with peroxidase-conjugated secondary antibodies. Following complete washes, proteins bands were visualized with DAB system (Sigma). All the experiments were repeated at least 3 times.
Indirect immunofluorescence
Subconfluent cells were detached and seeded on the 1 cm × 1 cm slides. Slides were fixed in pre-iced acetone for 10 min, then stained with primary antibody against MDM2 for 1 h followed by 45 min incubation with FITC-conjugated goat anti-rabbit IgG. After 3 times washing in PBS, cells were observed under fluorescence microscope.
In vitro growth rate analysis
All proliferation assays were carried out in quadruplicate wells in parallel 96-well microtiter plates. 5 × 103 cells were plated in each well added with LY294002 (25 μM) or vehicle. After cells were cultured for 24 h, 48 h, 72 h, 96 h and 120 h respectively, 20 μl MTT (5 mg/ml) was added and the cells were incubated for another 4 h. The medium was aspirated and the purple formazan crystals were solubilized in DMSO. Plates were read on Vmax Microplate reader, at a test wavelength of 490 nm.
Cytotoxicity assay
Proliferation cells (105/ml medium) in 96-well plates were treated in replicates of four wells with 2 μg/ml adriamycin combined with LY294002 (25 mM) or vehicle. Cells were incubated for a further 24 h, 48 h and 72 h, and then incubated with 20 μl of MTT (5 mg/ml) for 4 h at 37°C in a humidified 5% CO2 atmosphere. Purple formazan crystals were solublized in DMSO and the absorbance at 490 nm wavelength was read on Vmax Microplate reader. Cell viability was calculated according to the following formula.

Flow cytometry
Cells treated with adriamycin (2 μg/ml) combined with either PI3K pathway inhibitor LY294002 or vehicle were harvested at 24 h, 48 h and 72 h, after adriamycin treatment respectively. After fixed in citric acid buffer for 30 min, cell pellets were resuspended in 100 mg/l RNase and 10 mg/l PI diluted with PBS for 20 min. Apoptotic cell fraction was analyzed by FACScan cytometry. The cell cycle distribution of MCF7-neu3 cells treated with LY294002 or DMSO were analyzed by FACScan cytometry.
Irradiation condition
The cells were irradated by a Gammacell-40 model low-dosage-rate irradiation equipment, using 137Cs γ-ray. The radiation cavity cubage is 7.5 liter, central most radiancy is 0.94 Gy/min.
Immunohistochemistry
Our study comprised 229 patients with invasive ductal breast carcinoma, aged 29-89 years when operated on, in Huashan Hospital, Fudan University. In each case, mastectomy was performed and no treatment (radiotherapy or chemotherapy) was applied before operation. Specimens of tumor were fixed in 10% buffered formalin and embedded in paraffin blocks. Sections were cut to 4 μm thick. Microwave pre-treatment was used for better antigen retrieval and HER-2/neu, MDM2 and PCNA expression were stained with ABC (peroxidase-avidin-biotin complex) detection system.
Image and statistical analysis
Gel-pro analyzer4.0 software was used to quantify protein bands using β-actin as system reference. Data were expressed as Mean ± SD. The students' t-test was used to evaluate experimental data with SPSS 11.0 software. Values with P < 0.05 were considered significant.
RESULTS
HER-2/neu overexpression decreased the amount of wild-type p53 protein in MCF7 cells
To determine the effect of HER-2/neu overexpression on the amount of wild-type p53 protein, HER-2/neu gene inserted in pcDNA 3.0 was transfected into MCF7 breast tumor cells and high HER-2/neu expression clone (MCF7-neu3) was constructed. The clone was detected to have about 13 folds higher HER-2/neu gene expression than that in parental MCF7 and MCF7-pc3.0 (MCF7 transfected with pcDNA3.0) cells, and seemed as HER-2/neu overexpressing MCF7 cell clone. Western blot showed that the amount of p53 protein in MCF7-neu3 was only 40% of that in MCF7 (Fig. 1) cells, suggesting that over-expression of HER-2/neu decreased the amount of wild-type p53 protein in the MCF7 cells.
Overexpression of HER-2/neu decreased the amount of p53 protein in MCF7 cells. Photograph of a representative Western blot analysis of HER-2/neu, p53 and β-actin protein in MCF7, MCF7-pc3.0, MCF7-neu3 cells. Lane 1: MCF7 cells, Lane 2: MCF7-pc3.0 cells, Lane 3: MCF7-neu3 cells. 100 μg total protein was subjected to Western blot analysis to detect p53 while 30 μg total protein was used to detect HER-2/neu and β-actin.
Overexprssion of HER-2/neu induced nuclear localization of MDM2
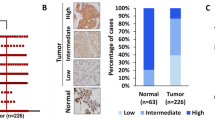
The main mechanism controlling the amount of p53 protein is posttranslational, through MDM2 mediated ubiquitination and degradation system 11. MDM2 is the direct regulator in this controlling system. To investigate whether HER-2/neu overexpression could affect MDM2 expression, Western blot and immunofluorescence were used to examine the expression and localization of MDM2 in MCF7 and MCF7-neu3 cells. Western blot assay showed no significant difference in the amount of MDM2 protein when total protein from the whole cell lysate was used for detection, while there was 2.1 times higher of MDM2 protein in MCF7-neu3 than that in MCF7 cells (Fig. 2A) when only nuclear protein was used. Using indirect immunofluorescence staining, we found that MDM2 in MCF7 cell was predominantly located in cytoplasm (Fig. 2B) while MDM2 in MCF7-neu3 cells was located in both nucleus and cytoplasm (Fig 2C). These results indicated that overexpression of HER-2/neu in MCF7 cells caused MDM2 protein translocation from cytoplasm to nucleus. To examine whether this is the same for in vivo experiments, we then detected the expression of HER-2/neu and MDM2 in breast cancer tissues of 80 cases. Immunohistochemistry results showed that HER-2/neu overexpression was found in 35 cases while MDM2 was detected in 73 cases. In the 35 cases of HER-2/neu overexpressing breast tumor tissues, MDM2 was mainly located in the nucleus or both cytoplasm and nucleus of these tumor cells, except for 3 negative cases and 7 cases in which MDM2 was mainly localized in cytoplasm. In the HER-2/neu non-over-expressing cases, MDM2 was prominently localized in cytoplasm in 23 cases, in 17 cases in both cytoplasm and nucleus or mainly in nucleus, and in other 4 cases was completely deprived (Tab. 1, Fig. 2). There was a great difference between subcellular localization of MDM2 in HER-2/neu overexpression and nonoverexpression groups (P < 0.01). These results indicated that overexpression of HER-2/neu caused translocation of MDM2 from cytoplasm to nucleus in human breast cancer tissues, which further confirmed our in vitro experiment.
The overexpression of HER2/neu caused MDM2 translocation from cytoplasm to nucleus in MCF7 cells. (A) Western blot indicates there was more nuclear MDM2 protein in MCF-neu3 than that in MCF7 cells while the total protein of MDM2 remained almost the same. (B) Immunofluorescence result showed MDM2 of MCF7 cells was mainly located in cytoplasm (200×). (C) Immuno-fluorescence result showed MDM2 of MCF7-neu3 cells was located in both nucleus and cytoplasm (200×). Tissue section from HER-2/neu negative breast cancer (D) showed MDM2 was mainly located in the cytoplasm of tumor cells (E), tissue section from HER-2/neu overexpression breast cancer (F) showed MDM2 was mainly located in the nucleus of tumor cells (G).
Blockage of PI3K pathway increased the amount of wild-type p53 but not mutant p53 protein in HER-2/neu overexpression breast tumor cells
Previous studies demonstrated that overexpression of HER-2/neu can delay the p53 dependent apoptosis through activation of PI3K pathway. From Western blot analysis, we noted that there was a 3 fold increase in the amount of p-Akt protein in MCF7-neu3 cells than that in parental MCF7 cells, while the total Akt protein remained unchanged in these two cell lines (Fig. 3A). To explore whether the activation of PI3K pathway participated in the down-regulation of p53 protein, we treated MCF7-neu3 cells with 25 μM LY294002, a specific inhibitor of the PI3K pathway. Western blot analysis showed that after 24h treatment with LY294002, the amount of wild-type p53 protein in MCF7-neu3 cells was 1.7 times higher, compared with that of the DMSO treated control cells. After 48 h incubation in the presence of LY294002, a 4.7 times increase in p53 protein was observed, compared with DMSO alone treated cells (Fig. 3B). Then we blocked PI3K pathway to determine its effect on the amount of mutant p53 protein in the MDA-MB-453 cells, which is with HER-2/neu overexpression and p53 gene mutation. Western blot result showed that LY294002 treatment for 24 h or 48 h had little effect on the amount of mutant p53 protein in MDA-MB-453 cells (Fig. 3C).
HER-2/neu overexpression decreased the amount of wild-type p53 protein via PI3K pathway. (A) Photograph of a representative Western blot analysis of Akt, p-Akt and β-actin in MCF7, MCF7-pc3.0 and MCF7-neu3 cells. (B) Western blot results showed blockage of PI3K pathway with LY294002 increased the amount of wild-type p53 protein in MCF7-neu3 cells. (C) Western blot analysis indicated blockage of PI3K pathway did not change the amount of p53 protein in breast tumor cell MDA-MB-453 that harbors mutant p53.
Overexpression of HER-2/neu promoted cell proliferation through activating of PI3K-Akt pathway
As shown in the cell growth curve (Fig. 4A), we found that the MCF7-neu3 cells grew faster (20% increased) than the parental MCF7 cells. Blockage of PI3K-Akt pathway with LY294002 decreased the cell growth rate on both MCF7-neu3 and parental MCF7 cells. FACS analysis showed that inhibiting PI3K pathway with LY294002 had an effect on the cell cycle distribution in MCF7-neu3 cells. As shown in Fig. 4B and 4C, the vehicle treated cells showed a distribution of 63.85% in G1/G0, 25.70% in the S phase, and 10.45% in G2/M. The treatment of LY294002 at a concentration of 25 μM increased the proportion of cells in G0/G1 phase to 77.64%, while the cells in S and G2/M phase decreased to 13.40% and 8.96% respectively. indicating a G1/S phase arrest. We also analyzed the immunohistochemistry results of HER-2/neu and PCNA expression in tissues of 229 cases of human breast cancer using Kendall's rank correlation method. The expression of HER-2/neu was determined according to the Herceptest standard (DAKO): nonexpression (−), mild expression (+), medium expression (++) and high expression (+++). The index of PCNA was calculated according to the percentage of positive cells: < 25% (+), 25-50% (++), 50-75% (+++) and > 75% (++++). Our data demonstrated that there was a close correlation of HER-2/neu expression with PCNA index (Tab. 2, P < 0.05). Using Western blot to determine HER2/neu and p-Akt in tissues of 6 cases with human breast cancer (Fig. 5), we also found a high correlation of HER-2/neu expression with the amount of p-Akt protein except one case, No.13034, in which coexisted low HER-2/neu and high p-Akt expression. All the above results suggested that HER-2/neu overexpression may accelerate the growth of human breast tumor cells through activating of PI3K-Akt pathway.
HER-2/neu overexpression increased cell proliferation rate via PI3K pathway activation. (A) MCF7-neu3 and MCF7 cells were cultured in RPMI1640 supplemented with 10% fetal bovine serum either with LY294002 or with DMSO for 24 h, 48 h, 72 h, 96 h, 120 h, then were detected for OD490 with MTT method, and growth curve was made. (B) FACS analysis of cell cycle distribution of MCF7-neu3 cells treated with DMSO for 48 h. (C) FACS analysis of cell cycle distribution of MCF7-neu3 cells treated with LY294002 for 48 h. There were G1-S phase arrest when MCF7-neu3 cells were treated with LY294002 (25 μM).
Blocking PI3K pathway reversed cancer cell resistance to γ-irradiation caused by HER-2/neu overexpression
When treated with 8Gy γ-irradiation, MCF7-neu3 cells showed higher surviving fraction than parental MCF7 cells. Combined treatment of LY294002 (25 μM) with γ-irradiation resulted in the reduction of surviving fraction in both MCF7-neu3 and MCF7 cells (Fig. 6).
Blocking PI3K pathway increased the sensitivity to adriamycin in MCF7-neu3 cells but not in MDA-MB-453 breast tumor cells
FACS analysis results revealed no apoptosis (with apoptotic cell fraction less than 1%) when MCF7-neu3 cells were treated with LY294002 alone (data not shown). Combining LY294002 with adriamycin caused a increase in the apoptotic cell fraction compared with the DMSO treated control group (Fig. 7A, P < 0.05). Cytotoxicity experiment results indicated that blocking PI3K pathway with LY294002 led to the reduction of cell viability after in MCF7-neu3 cells when exposed to adriamycin treatment (Fig. 7B). However, blocking PI3K pathway with LY294002 did not show any significantly effect on the cell viability in MDA-MB-453 cells (in which p53 is mutated) after adriamycin treatment (Fig. 7C).
Blockage of PI3K pathway increased sensitivity to adriamycin in MCF7-neu3 cells with wild-type p53 but not in MDA-MB-453 cells in which p53 is mutated. (A) FACS analysis results indicated blocking PI3K pathway with LY294002 increased the apoptotic cell fraction induced by adriamycin in MCF7-neu3 cells. MCF7-neu3 cells were treated with adriamycin (2 μg/ml) combined with either LY294002 (25 μM) or DMSO. When cultured for additional 24 h, 48 h, 72 h, cells were collected for FACS analysis. (B) Cytotoxicity experiments showed inhibiting PI3K pathway resulted in reduction of cell viability after adriamycin treatment in MCF7-neu3 cell. MCF7-neu3 cells were treated with adriamycin combined with either LY294002 (25 μM) or DMSO. When cultured for additional 24 h, 48 h, 72 h, MTT method was used to detect OD490, and the cell viability was calculated. (C) Cytotoxicity experiments showed that inhibiting PI3K pathway did not significantly affect cell viability after adriamycin treatment in MDA-MB-453 cells. MDA-MB-453 cells were treated with adriamycin combined with either LY294002 (25 μM) or DMSO. When cultured for additional 24 h, 48 h, 72 h, MTT method was used to detect OD490, and the cell viability was calculated.
DISCUSSION
Amplification or overexpression of HER-2/neu occurs in about 30% of human breast cancers, and was correlated with tumor progression and poor prognosis for some complicated reasons not very clearly known so far 2,3,4. Previous studies have revealed that overexpression of HER-2/neu can affect the cell cycle or apoptosis regulating gene, such as p21 , p27 , Bad , caspase-9 and IKK-α, thereby promoting cell survival 12,13,14. However, little was known about whether the overexpression of HER-2/neu can affect p53 gene expression. In this study, we used high amount of total cellular protein for the detection of wild-type p53 protein and found there was a reduced expression of 53 protein in MCF7-neu3 cells than that in parental MCF7 cells. Because MCF7 is a cell line expressing wild-type p53, these results indicated that overexpression of HER-2/neu may decrease the amount of wild-type p53 protein in breast tumor cells.
The main mechanism controlling activity and quantity of p53 protein level is through MDM2-dependent ubiquitination system. MDM2, an ubiquitin ligase for p53, plays a central role in the regulation of the stability of p53 and served as a good substrate for Akt 9,11,15. It binds directly to p53 and promotes its ubiquitination and subsequent degradation by the proteasome. Tumors with MDM2 overexpression or having MDM2 induced by other factors do not have normal p53 function, because wild-type p53 protein in these tumors degrades more quickly. Although MDM2 degrades p53 in cytoplasm, it is in nucleus for MDM2 to bind p53 and repress p53-mediated apoptotic activity. When MDM2 is sequestrated in the cytoplasm by PTEN or other factors, its p53 binding and inhibitory function are blocked, and an increase of p53 stability can be seen 16. In the current study, we did not find that overexpression of HER-2/neu could induce a higher MDM2 expression. However, an increased MDM2 nuclear localization in MCF7 cells was observed. This may account for the lower p53 protein in HER-2/neu overexpression human breast cancer cells 17. This relevance was further tested in tissues of 80 cases with human breast cancer using immunohistochemistry staining. And it was found that in HER-2/neu overexpressing breast cancer tissues MDM2 was mainly located in nucleus or in both nucleus and cytoplasm, whereas in HER-2/neu nonoverexpression breast cancer tissues MDM2 was mainly located in the cytoplasm of tumor cells. Our results strongly suggested that HER-2/neu could induce MDM2 to translocate from cytoplasm to nucleus both in vivo and in vitro.
It was well documented that PI3K pathway played an important role in preventing cells from undergoing apoptosis and contributed to the pathogenesis of malignancy 18. Activation of this pathway was also reported to be able to delay the p53-mediated apoptosis. We then wondered whether activation of this pathway contributed to the change of the amount of p53 protein. As suggested by Western blot analysis, the overexpression of HER-2/neu led to PI3K pathway activation and reduced the amount of p53 protein in human breast cancer cell line MCF7. Blocking PI3K pathway with LY294002, a specific PI3K pathway inhibitor, could greatly increase the amount of p53 protein in MCF7-neu3 cells. Since MCF7 is a cell line containing wild-type p53, we then tried to detect whether mutant p53 protein would also be regulated through this pathway. To test this, MDA-MB-453 breast tumor cells was used, in which p53 is mutated and PI3K pathway is activated by HER-2/neu overexpression. However, the amount of mutant p53 protein in MDA-MB-453 cells did not change when cells were incubated with LY294002 for 24 h or 48 h.
Previous studies showed MDM2 was an important target of Akt to regulate the amount of wild-type p53 protein, however, researches from other groups showed divergent effect of Akt activation on MDM2. Some of them showed that Akt phosphorylated MDM2 at Ser166, Ser186 19,20, increased its nuclear localization, enhanced its interaction with p300, inhibited its intereaction with p19ARF, thus increasing p53 degradation; others showed that Akt phosphorylated MDM2 at Ser188 21, resulting in the change of its self-ubiquitination. The different results may be due to that different cell lines with different genetic backgrounds were employed, which may also reflect the complicated regulation mechanism in the cell signaling pathways. The findings of our experiment is consistent with what was reported by Mayo LD and Zhou BP 19,20, and explains the reason of lower p53 protein in the HER-2/neu overexpressing breast cancer cells. As to the mutant p53, which was modified in amino acid sequence, may not be regulated through the MDM2 mediated ubiqutination and degradation system, and the half-life of mutant p53 is much longer (2 h or longer) than wild-type p53 protein (5-15 min).
Wild-type p53 is critical in regulating cell cycle arrest and apoptosis 9,22. p53 gene mutation or deletion and loss of p53 normal function lead to many kinds of human tumors. Our work demonstrated that HER-2/neu overexpression induced MDM2 nuclear localization and reduced the amount of wild-type p53 protein in human breast tumor cells. This may account for the poor prognosis and therapeutic resistance in the HER-2/neu overexpression tumors, at least in those cases containing wild-type p53 gene.
In this study, we also showed that HER-2/neu overexpression in MCF7 cells promotes proliferation through the activation of the PI3K-Akt pathway. Inhibiting PI3K pathway caused G1-S phase arrest and decreased the cell proliferation rate in MCF7-neu3 cells, which was consistent with the other researcher's report that elevated amount of wild p53 protein could cause cell cycle G1-S phase arrest 23. We further examined whether HER-2/neu overexpression activated PI3K pathway in human breast cancer tissues using Western blot analysis. Our results showed a good relevance between HER-2/neu over-expression and high amount of p-Akt. By analyzing the results of immunohistochemistry detection in 229 cases of human breast cancer tissues, we found HER-2/neu overexpression was correlated with higher PCNA index. Since PCNA is the most common index used for reflecting cell proliferation, our results both in vivo and in vitro consistently proved that HER-2/neu overexpression can promote cell proliferation through PI3K pathway.
p53 is a key regulator in determining cell response to γ-irradiation and chemotherapeutic drugs 15,21. Since HER-2/neu decreased wild p53 protein level through PI3K pathway, we then detected whether HER-2/neu over-expression could affect drug or irradiation sensitivity in human breast cancer cells through this pathway. Our results showed that there was a decrease in sensitivity to γ-irradiation in MCF7-neu3 cells overexpressing HER-2/neu than that in parental MCF7 cells. Blocking PI3K pathway can decrease the cell viability after γ-irradiation. This result suggested that the decrease of wild-type p53 protein via PI3K pathway induced by HER-2/neu overexpression also contributed to the γ-irradiation resistance.
Although chemotherapy for the treatment of cancer was introduced into the clinic more than 50 years ago, chemo-therapeutic resistance still remains a common clinical problem in tumor therapy. The effectiveness of cancer therapy has suffered from a range of confounding factors, and the apoptotic capacity of the cancer cells is pivotal in determining the response to chemotherapeutic agents. Adriamycin, a topoisomerase II inhibitor, is commonly used in chemotherapy of human breast cancer. However, the use of adriamycin was to some extent restrained because of its wide tissue toxicity. So, the question of how to increase the sensitivity to adriamycin become a very significant problem in tumor therapy. The effect of adriamycin relies on the p53 gene status, cells with p53 deficient or mutated would be more resistant to adriamycin. In the present study, we found that blocking PI3K pathway increased the amount of wild-type p53 protein and the sensi-tivity to adriamycin in MCF7-neu3 cells which contains wild-type p53, an effect that was absent in the MDA-MB-453 cells expressing mutant p53. These observations clearly suggested that blocking PI3K pathway would be an effective way for manipulating anti-tumor therapeutic strategies in breast tumor with both HER-2/neu over-expression and wild-type p53 gene. The relationship of cell signaling pathway and chemical drug resistance has been largely studied recently. It was widely accepted that PI3K-Akt pathway activation was correlated with invasive behavior of tumor cells and resistance to adriamycin induced apoptosis 6,24. Our study showed inhibiting PI3K pathway can increase the sensitivity to adriamycin in HER-2/neu overexpression breast tumor cells with wild-type p53, but not in MDA-MB-453 cells with mutant p53, strongly suggesting that wild-type p53 is also required for the proapoptotic effects of down-regulation of p-Akt activity. Some other recent studies also revealed the significance of inhibiting PI3K pathway or recovery of tumor suppressor gene, PTEN function in modulating chemotherapeutic sensitivity and tumor angiogenesis in some kinds of tumor, and some of the above effects also depended on normal p53 function 25,26. These works further supported that both p53 and p-Akt can regulate adriamycin sensitivity, and p53 played an important role in the formation and treatment of cancer through regulating of PI3K pathway 27. All of these prompted us to think about the development of novel combination of inhibitors of the PI3K-Akt pathway in the treatment of HER-2/neu overexpresssion breast cancer, at least in those cases with wild-type p53 gene.
Taken together, the results of this study demonstrated that HER-2/neu overexpression downregulated wild-type p53 protein, increased cell proliferation and decreased the sensitivity to chemotherapeutic drug adriamycin and γ-irradiation through activation of PI3K pathway. In addition, the ability to increase adriamycin sensitivity by blocking PI3K pathway relied on normal p53 function. Our work shed some new light on revealing the molecular mechanism of human breast cancer progression, and may have implication for the future therapy in HER-2/neu over-expressing breast tumors.
References
Schechter AL, Hung MC, Vaidyanathan L, et al. The neu gene: an erbB-homologous gene distinct from and unlinked to the gene encoding the EGF receptor. Science 1985; 4717:976–78.
Bacus SS, Ruby SG, Weinberg DS, et al. HER-2/neu oncogene expression and proliferation in breast cancers. Am J Pathol 1990; 137:103–11.
Berchuck A, Kamel A, Whitaker R . Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Res 1990; 50:4087–91.
Borg A, Tandon AK, Sigurdsson H . HER-2/neu amplification predicts poor survival in node-positive breast cancer. Cancer Res 1990; 50:4332–7.
Zhou BP, Hu MC, Miller SA, et al. HER-2/neu blocks tumor necrosis factor-induced apoptosis via the Akt/NF-kappaB pathway. J Biol Chem 2000; 275:8027–31.
Sabbatim P, McCormick F . Phosphoinositide 3-OH kinase (PI3K) and PKB/Akt delay the onset of p53-mediated, transcrptionally dependen apoptosis. J Biol Chem 1999; 274:24263–69.
Levine AJ, Momand J, Finlay CA . The p53 tumour suppressor gene. Nature 1991; 351:453–6.
Hollstein M, Sidransky D, Vogelstein B Harris CC . p53 mutations in human cancers. Science 1991, 253:49–53.
Woods DB, Vousden KH . Regulation of p53 function. Exp Cell Res 2001; 264:56–66.
Zheng L, Ren JQ, Chen Q, et al. Preparation of Anti-HER2/neu monoclonal antibody and its inhibiting ablility on the growth of breast carcinoma cells. Fudan Univ J Med Sci 2003; 30:151–3.
Momand J, Wu HH, Gasgupta G . MDM2-master regulator of the p53 tumor suppressor protein. Gene 2000; 242:15–29.
del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez, G . Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 1997; 278:687–9.
Zhou BP, Liao Y, Xia W, et al. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol 2001; 3:245–52.
Cardone MH, Roy N, Stennicke HR, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science 1998; 282:1318–21.
Vogelstein B, Lane D, Levine AJ . Surfing the p53 network. Nature 2000, 408:307–10.
Mayo LD, Dixon JF, Podsypania K, et al. PTEN protects p53 from Mdm2 and sensitizes cancer cells to chemotherapy. J Bio Chem 2002, 277:5484–89.
Roth J, Dobbelstein M, Freedman DA, Shenk T, Levine AJ . Nucleo-cytoplasmic shuttling of the hdm2 oncoprotein regulates the levels of the p53 protein via a pathway used by the human immunodeficiency virus rev protein. Embo J 1998; 17: 554–64.
Datta SR, Brunet A, Greenberg ME . Cellular survival: a play in three Akts. Genes Dev 1999; 13:2905–27.
Zhou BP, Liao Y, Xia W, et al. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol 2001; 3:973–82.
Mayo LD, Donner DB . A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci 2001; 98:11598–603.
Feng JH, Tamaskovic R, Yang Z, et al. Stabilization of Mdm2 via decreased ubiquitination is mediated by protein kinase B/Akt-dependent phosphorylation. J Biol Chem 2004; 279:35510–7
Vousden KH . p53: death star. Cell 2000; 5:691–4.
Bhatia U, Danishefsky K, Traganos F, Darzynkiewicz Z . Induction of apoptosis and cell cycle-specific change in expression of p53 in normal lymphocytes and MOLT-4 leukemic cells by nitrogen mustard. Clin Cancer Res 1995; 1:873–80.
Takeuchi K, Ito F . Suppression of adriamycin-induced apoptosis by sustained activation of the phosphatidylinositol-3′-OH kinase-Akt pathway. J Biol Chem 2004, 279:892–900.
Su JD, Mayo LD, Donner DB, Durden DL . PTEN and phosphatidylinositol 3′-kinase inhibitors up-regulate p53 and block tumor-induced angiogenesis: evidence for an effect on the tumor and endothelial compartment. Cancer Res 2003; 63:3585–92.
Fraser M, Leung BM, Yan X, et al. p53 is a determinant of X-linked inhibitor of apoptosis protein/Akt-mediated chemoresistance in human ovarian cancer cells. Cancer Res 2003; 63: 7081–7.
Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C . PI3K/Akt and apoptosis: size matters. Oncogene 2003; 22: 8983–8.
Acknowledgements
We thank Dr. Ena Wang (NIH, New York) and Dr. Jin Ming Yang (Cancer Institute of New Jersery) for improving our use of the English language in this manuscript. We also thank Dr. Chen Yang for his kind gift of the CelLyticTM NuclearTM Extraction Kit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
ZHENG, L., REN, J., LI, H. et al. Downregulation of wild-type p53 protein by HER-2/neu mediated PI3K pathway activation in human breast cancer cells: its effect on cell proliferation and implication for therapy. Cell Res 14, 497–506 (2004). https://doi.org/10.1038/sj.cr.7290253
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.cr.7290253
Keywords
This article is cited by
-
Interplay between TGF-β signaling and receptor tyrosine kinases in tumor development
Science China Life Sciences (2017)
-
The cholesterol metabolite 27-hydroxycholesterol regulates p53 activity and increases cell proliferation via MDM2 in breast cancer cells
Molecular and Cellular Biochemistry (2015)
-
The carboxy-terminal domain of connexin 43 (CT-Cx43) modulates the expression of p53 by altering miR-125b expression in low-grade human breast cancers
Cellular Oncology (2015)
-
Aberrant miR-215 expression is associated with clinical outcome in breast cancer patients
Medical Oncology (2014)
-
Inhibition of tumor promoting signals by activation of SSTR2 and opioid receptors in human breast cancer cells
Cancer Cell International (2013)