ABSTRACT
Expression of the adhesion molecules, ICAM-1, VCAM-1, NCAM, CD44, CD49d (VLA-4, α chain), and CD11a (LFA-1, α chain) on mouse oocytes, and pre- and peri-implantation stage embryos was examined by quantitative indirect immunofluorescence microscopy. ICAM-1 was most strongly expressed at the oocyte stage, gradually declining almost to undetectable levels by the expanded blastocyst stage. NCAM, also expressed maximally on the oocyte, declined to undetectable levels beyond the morula stage. On the other hand, CD44 declined from highest expression at the oocyte stage to show a second maximum at the compacted 8-cell/morula. This molecule exhibited high expression around contact areas between trophectoderm and zona pellucida during blastocyst hatching. CD49d was highly expressed in the oocyte, remained significantly expressed throughout and after blastocyst hatching was expressed on the polar trophectoderm. Like CD44, CD49d declined to undetectable levels at the blastocyst outgrowth stage. Expression of both VCAM-1 and CD11a was undetectable throughout. The diametrical temporal expression pattern of ICAM-1 and NCAM compared to CD44 and CD49d suggest that dynamic changes in expression of adhesion molecules may be important for interaction of the embryo with the maternal cellular environment as well as for continuing development and survival of the early embryo.
Similar content being viewed by others
INTRODUCTION
Cellular adhesion molecules (CAMs) play an important role in defining cellular shape and degree of contact with neighbouring cells. They have a role in generating and maintaining cell junctions and constitute an extensive cell-cell and cell-matrix network. Changes in expression of adhesion molecules are involved in immune responses, lymphocyte homing, platelet aggregation, carcinoma metastasis, and embryo development, during which cell-cell interactions mediate the initial steps in lineage segregation1, 2, 3. As development proceeds, embryonic tissues express developmentally regulated, complex repertoires of cell-cell adhesion receptors, providing a potential for recognition and signalling specificity2, 4, 5.
Cellular adhesion molecules can be classified into five groups, according to their molecular structure. These include cadherins, the immunoglobulin superfamily (IgSF), integrins, selectins and the homing-associated cell adhesion molecule (HCAM)1.
Although cadherins are thought to be primarily responsible for the cell-cell adhesion seen in early vertebrate embryos4, 6, a variety of integrin subunits have been detected on mouse and human pre- and peri-implantation embryos, including α1, α3, α5, α6, α7, αV, β1, β3, β4, β57, 8, 9], suggesting that these molecules may play a role in embryonic development.
In the adult much of the functionality of IgSF molecules like ICAM-1 (CD54) and VCAM-1 (CD106) clearly depends on expression of their integrin receptors, LFA-1 (αL/β2, CD11a/CD18) and VLA-4 (α4/β1, CD49d/CD29), respectively. Expression of VCAM-1 in early pregnant decidua10 therefore begs the question whether VLA-4 is expressed on pre- and peri-implantation embryos. However, little is known about expression of these cell-based integrins during early embryological development.
Expression of the HCAM, CD44, although reported on human embryos11 has not been examined in the murine embryo. Its common ligand is the extracellular matrix molecule, hyaluronate, widely expressed throughout embryonic and adult tissues. CD44 is important in cellular migration, especially in the immune response, and metastasis, both of which have features in common with embryological development.
We have shown that murine embryonic stem cells (ES) express high levels of IgSF molecules12. These declined rapidly upon chemically-induced differentiation, or upon withdrawal of leukaemia inhibitory factor, the cytokine most commonly used to maintain the totipotency of ES cells. This occurred before morphological signs of differentiation, raising the possibility that high level CAM expression may define the undifferentiated phenotype, with changes heralding the onset of differentiation.
Therefore, to determine whether expression of IgSF molecules is a normal feature of early embryonic cells, we determined the relative quantitative expression of a range of immunologically important CAMs at particular developmental stages throughout the pre- and peri-implantation period. The CAMs studied included the IgSF molecules, ICAM-1, NCAM (CD56) and VCAM-1 and their complementary cell-based integrin receptors, CD11a and CD49d, as well as CD44.
For each molecule expressed we found a consistent, temporally regulated pattern of expression from oocyte to blastocyst outgrowth, suggesting a functional involvement in interactions with the maternal endometrial environment as well as embryonic development and survival at these developmental stages.
MATERIALS AND METHODS
Animals
Random-bred outbred female Swiss albino mice of Quackenbush strain (QS) (6 to 10 weeks old) (Sydney University, Sydney, NSW, Australia) were specifically chosen for these experiments. The adhesion molecules investigated are essentially monomorphic. Furthermore, since this study was based on large sample sizes, we took the view that any genetic variation that might potentially be encountered with outbred animals, which could result in quantitative and/or qualitative (e.g., as a result of unknown polymorphic differences) differences in molecule expression between animals, would average out. We felt that the results from outbred mice would thus be more widely applicable than if this study were done in a single inbred strain of mouse. QS mice were superovulated by an intraperitoneal injection of 10 IU of pregnant mare's serum gonadotropin (PMSG; Intervet, Sydney, NSW, Australia). This was followed 48 hours later by an injection of 10 IU human chronic gonadotropin (hCG; Intervet, Sydney, Australia) immediately after which, unless ovulated oocytes were required, females were caged with QS males. Females were checked for the presence of a copulation plug in the vagina the morning after mating (day 1 post coitum [pc]). Mice were sacrificed at different times after hCG injection. All animals were used according to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, and all procedures were approved by the Institutional Animal Care and Ethics Committee.
Solutions for embryo collection and embryo culture
The medium used for oocyte and embryo collection was HEPES-buffered synthetic human tubal fluid (HHTF) medium13, 14, which was supplemented with 3 mg bovine serum albumin per ml (BSA; CSL Limited, Victoria, Australia) (HHTF-BSA). Embryo washing medium used for indirect immunofluorescent labelling was phosphate-buffered saline (Sigma, BioSciences, St. Louis, MO., USA) containing 0.1% BSA (PBS-BSA). Hyaluronidase solution consisted of HHTF-BSA in which hyaluronidase, 300 units/ml (Sigma Chemical Co., St Louis, USA), was dissolved. Blastocyst culture medium was Dulbecco' Modified Eagle' Medium (DMEM, Cytosystems Pty Ltd. Australia), supplemented with 10% bovine foetal calf serum (FCS), 74 units/ml Streptomycin and 980 units penicillin G per ml.
Monoclonal antibodies
Primary rat monoclonal antibodies against plasma membrane CAMs were used as follows. Anti-mouse ICAM-1 (CD54), hybridoma supernatant, (clone: YN1/1.7.4, ATCC# CRL 1878), anti-mouse VCAM-1 (CD106) purified (clone: 492 [MVCAM.A], Pharmingen, San Diego, USA), anti-mouse NCAM (CD56) purified (clone: H28.123, Immunotech, S.A. Australia), anti-mouse CD44, purified (clone: IM7, Pharmingen, San Diego, USA), anti-mouse VLA-4, α4-chain (CD49d) purified (clone: 9C10 [MFR4.B], Pharmingen, San Diego, USA), anti-mouse LFA-1, αchain (CD11a) purified (clone: 2D7, Pharmingen, San Diego, USA). Affinity-purified polyclonal normal rat IgG, (Southern Biotechnology Associates, Inc. Birmingham, USA) and affinity-purified, fluorescein isothiocyanate (FITC)-conjugated sheep-anti-rat IgG, (Silenus, Hawthorn, Australia) were used for non-specific control labelling and secondary antibody labelling, respectively.
Embryo collection
Uterine horns with oviducts were placed in 0.5 ml HHTF-BSA medium in a 35 mm petri dish (Falcon 3001, Becton Dickinson, New Jersey, USA). Embryos were flushed out, using HHTF-BSA medium, either from the oviduct or uterine horn, depending upon the stage of development. To obtain 1-cell embryos or unfertilised oocytes, cumulus cell complexes were placed in 1 ml hyaluronidase solution in an organ tissue culture dish (Falcon 3037, Becton Dickinson, New Jersey, USA) for approximately 20 seconds at 37°C to disperse the cumulus cells. Single-cell embryos or oocytes thus released were then washed once in 1ml HHTF-BSA medium and twice in 3 ml HHTF-BSA medium.
Embryos at various defined developmental stages were selected based on optimal morphological and culture criteria. To minimise differences between individual animals, at least three mice were used in each experiment, and approximately 10 selected embryos were randomly allocated to each group.
Embryo culture
To obtain blastocyst outgrowth freshly isolated blastocysts were cultured in groups of 10 for 96 h15 on a coverslip (13 mm in diameter, Mediglass, Australia) placed in a 24-well plate (Nunclon 1 43982, Nunc, Denmark) in 2 ml DMEM, pre-equilibrated under standard culture conditions (SCC; i.e., humidified 95% air with 5% CO2 at 37°C). To obtain hatched blastocysts, freshly isolated blastocysts were cultured under the above conditions for approximately 48 h.
Immunofluorescent antibody labeling
Expression of ICAM-1, VCAM-1, NCAM, CD44, CD49d and CD11a on cells of murine embryos at different stages was determined by indirect immunofluorescence labelling. Freshly collected embryos of different stages were washed twice in HHTF-BSA and fixed in fresh 2% paraformaldehyde (pH 7.4) in PBS at room temperature (25°C) for 1 h. Embryos were then washed three times in 2 ml PBS-BSA for 15 min and blocked with 30% sheep serum (from the same source as the secondary antibody) diluted in PBS-BSA, for 30 min at 25°C. After three further washes in 2 ml PBS-BSA for 15 min, they were randomly divided into equal groups and incubated in a drop of 10 μl of primary antibody at a concentration of 25 μg/ml (0.25 μg total antibody per sample) in PBS-BSA (except anti-ICAM-1, which was a hybridoma supernatant) overnight at 25°C. These drops were in a 60-well tissue culture plate (LUX 5260, Nunc. Inc., Naperville, IL, USA), covered by approximately 10 μl heavy paraffin liquid (BDH Laboratory Supplies, Poole, England). After this time, embryos were washed three times in 2 ml PBS-BSA for 15 min and then incubated in a 60-well tissue culture plate with 10 ml secondary antibody (sheep-anti-rat IgG-FITC) diluted 1:20 in PBS-BSA for 1 h at 25°C Following this, embryos were given three final washes in 2 ml PBS-BSA for 15 min. Negative controls consisted of fixed embryos, incubated in normal rat Ig, followed by FITC-conjugated secondary antibody, or incubated in FITC-conjugated secondary antibody only, or in PBS-BSA only, were included in all experiments. This was to exclude the possibility of false-positive fluorescence from non-specific binding of primary and/or secondary antibodies, or from paraformaldehyde-induced autofluorescence.
The concentration of ICAM-1 antibody in the hybridoma supernatant used in these experiments, as estimated from Protein G yields, was < 25μg/ml, i.e., lower than that of the concentration of the non-specific isotype control antibodies used. At these concentrations it was possible to show that expression of ICAM-1 on the surface of pre and peri-implantation murine embryos was down-regulated over time, and absent by the fresh blastocyst stage (See results). To ensure saturation labeling, each primary antibody was titrated separately at 1, 0.5, 0.25, 0.1 and 0.05 μg per sample on samples of 105 adult cells of various types, expressing high levels of each antigen. A second, sheep-anti-rat IgG-FITC, as above, was used to label the first antibody on these cells and the level of fluorescence of each sample was then measured by flow cytometry. Thus ICAM-1 and VCAM-1 antibodies were titrated on murine endothelial cells, induced by interferon-g for 48 h. NCAM, CD44, CD49d and CD11a were all titrated on spleen cell populations from mice injected intraperitoneally with 106 plaque-forming units of West Nile virus (Sarafend) 3 and 6 days previously to generate an anti-viral immune response (data not shown). Since all antibodies were titrated to obtain maximal labelling on high-expressing cells prior to embryo labelling, it is reasonable to assume that the concentration of antibody used was in all cases sufficient to label the accessible parts of the embryos quantitatively.
Labelled oocytes, morulae and blastocyst outgrowths were mounted in 30% 1,4 diazabicyclo-[2.2.2] octane (DABCO) anti-fade glycerol (BDH Chemicals, Victoria, Australia) in PBS-BSA16 and examined under a fluorescence microscope. Fresh and hatched blastocysts were mounted in PBS-BSA only before examination.
Epifluorescence microscopy and photometry
Mounted slides with labelled embryos were evaluated immediately by epifluorescence microscopy under a Nikon fluorescence microscope with a U.V. filter block with a BV-1A lens. The total intensity of emitted green fluorescence was quantified using a Nikon P1 photometer. Photographs were taken with Kodak Ektachrome 1600 professional slide film, using fixed times and apertures for all images.
To quantitate fluorescence intensity, images were acquired under the same conditions with identical microscope and photometer settings for all embryos and groups in each experiment. The mean value of the normal rat Ig non-specific control for each group was normalised to a value of 1, and the fluorescence intensity value of individual fluorescent antibody-labelled embryos in the same experiment was then multiplied by the normalization factor.
From oocyte to morula stages, total fluorescence, i.e., the fluorescence emitted by the whole embryo, was measured. However, in later embryos, because of the 3-D complexity of the labelling pattern in both unhatched and hatched blastocysts and the unavailability of confocal microscopy, only limited quantitative epifluorescence observations were made, as follows. In blastocysts, the fluorescence of trophectoderm away from the ICM was measured. In blastocyst outgrowths, 2-3 representative areas of outgrown primary trophoblast giant cell monolayer per embryo were measured. Separate measurements were done on 10-12 embryos (from at least three mice) cultured on the same coverslip. Measurements from three separate experiments obtained in this way were amalgamated.
Although we were able only to measure total fluorescence, from a functional point of view, it seems more likely that the fluorescence value per unit cell surface area (which is proportional to the number of adhesion molecules present) of is more meaningful than total embryonic expression. We therefore also calculated total embryonic fluorescence data as a function of calculated cell surface area for embryos up to the morula stage as a comparison. Areas were estimated as follows; the longest, shortest and intermediate diameters of each of the individual cells from each of 4 embryos at each relevant stage were measured using a scale, photographed at the same magnification as the embryos. The average value for cell radius at each stage was then calculated and used in the standard formula, Area = 4πr2, making the crude assumption that each cell was spherical. This value was then multiplied by the number of cells in the embryo. Compacted 8-cell embryos and morulae were treated as 'single' cells, since the fluorescent label did not penetrate beyond the outer surface of the embryos at these stages. The radii were therefore calculated on the average total diameters of whole embryos from these later stages.
Statistical analysis
Fluorescence intensities of test groups were compared using a one-way analysis of variance (ANOVA) followed by a Tukey multiple comparison post test to estimate the statistical significance of changes in CAM expression at different stages of pre-implantation embryonic development. This was calculated using SPSS statistical analysis package, version 7.5 for Windows. Each experiment was repeated on separate occasions at least three times. Each of the values shown represent an average of individual values measured at the same embryological stage from different experiments.
RESULTS
Embryos were stained for ICAM-1, VCAM-1, NCAM, CD44, CD49d (VLA-4, α chain) and CD11a (LFA-1, α chain) at sequential stages during the pre-implantation period. Of these molecules, VCAM-1 and CD11a were consistently undetectable in our hands at all stages of preimplantation embryonic development (data not shown). Initial values were based on total fluorescence and were used to calculate fluorescence per unit cell surface area of the embryo, since expression per unit area potentially gives a functional indication of CAM expression and also controls for different embryo sizes.
ICAM-1 expression
ICAM-1 was expressed at the highest levels at the oocyte stage (Fig 1, panels, D-F; Fig 2a). During development from oocyte to blastocyst, expression of ICAM-1 decreased significantly (P < 0.001; ANOVA with Tukey post test). Expression remained undetectable on trophectoderm and trophoblast of hatched and outgrown blastocysts, respectively (Fig 2a), as well as the ICM of blastocyst outgrowths (data not shown).
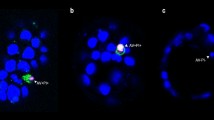
Photomicrographs showing expression of ICAM-1 (CD54) (Panels D-F) and NCAM (CD56) (Panels G-I) on representative preimplantation embryos from oocyte, 2-cell and compacted 8-cell stages. Panels A-C show relevant isotype control labelling at equivalent stages. The bar in panel I represents 50 μM. The magnification is identical in all panels.
Line graphs showing the change in ICAM-1 (CD54) (a), NCAM (CD56) (b), CD49d, the α4 chain of integrin VLA-4 (c), and CD44 (d) expression on the surface of oocyte, 1-cell, 2-cell, uncompacted (u/c) and compacted (c) 8-cell embryos, morula, unhatched (u/h), hatched (h) and outgrown (o/g) blastocysts. The Y1 ordinate represents total fluorescence measured by photometry at each stage. These values are represented in the solid line graph. The Y2 ordinate represents total fluorescence corrected for the calculated exposed cell surface area of the embryo up to the morula stage. These values are represented in the broken line graph. Each value represents the average of measurements from at least 10 embryos±standard error (see Materials and Methods).
NCAM expression
Relative intensity of fluorescence of NCAM was less than that observed for ICAM-1. Like ICAM-1, however, staining was brightest on oocytes (Fig 1, panels G-I; Fig 2b). This was significantly reduced by the blastocyst stage (P < 0.001; ANOVA with Tukey post test) when it was undetectable on trophectoderm. Similar to ICAM-1, NCAM was undetectable on both primary trophoblast giant cells (Fig 2b) and ICM of the blastocyst outgrowth (data not shown).
CD49d expression
Expression of CD49d was detected at all stages of embryonic development investigated except blastocyst outgrowth (Fig 3a, panels I-L; Fig 2c). From oocyte to 2-cell stage, expression of CD49d decreased significantly (P < 0.001; ANOVA with Tukey post test). On both uncompacted and compacted 8-cell stages, fluorescence intensity of CD49d labelling was significantly greater than that on the 2-cell stage (P = 0.002 and P = 0.005, respectively; ANOVA with Tukey post test) and reached a similar level to that observed at the single-cell stage. From compacted 8-cell to morula stage, expression of CD49d decreased significantly (P < 0.001; ANOVA with Tukey post test) but thereafter maintained a plateau of positive expression on trophectoderm of both unhatched and hatched blastocysts, disappearing on both primary trophoblast giant cells (Fig 2c) (hatched blastocysts compared with trophoblast giant cell outgrowth P= 0.024; ANOVA with Tukey post test) and ICM of blastocyst outgrowths (data not shown).
The pattern of CD49d labelling on the trophectoderm of hatching embryos was generally diffuse. In more than 25% (n=86) of hatched blastocysts, fluorescence was observed to be maximal over the area of trophectoderm furthest away from the ICM (Fig 3a, panel L). However, the possibility that these areas of maximal fluorescence were randomly distributed over trophectoderm could not be excluded.
CD44 expression
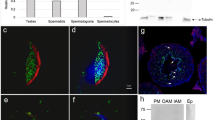
Expression of CD44, like CD49d, was detected at all stages of embryonic development investigated except blastocyst outgrowth. Levels decreased significantly from oocyte to uncompacted 8-cell stage (P < 0.001; ANOVA with Tukey post test). This was followed by a significant increase from uncompacted to compacted 8-cell and morula stages (P = 0.005 and P = 0.002, respectively; ANOVA with Tukey post test), with expression returning at this time to the higher levels seen at the 2-cell stage. Expression then declined significantly from morula to unhatched blastocyst (P < 0.001; ANOVA with Tukey post test) and appeared to increase once again on hatched blastocyst (Fig 3a, Panels E-H; Fig 2d), although this was not statistically significant.
We also cultured blastocysts in vitro for 48 h. At this time blastocysts which had begun to hatch, but were not yet attached to the substratum, were labelled for CD44. Intriguingly, we found some 50% (n=8) of hatching blastocysts labelled for CD44 most intensely on the trophectoderm at the site directly adjacent to the hatching aperture in the zona pellucida (Fig 3b, panel B). With complete hatching, CD44 labelling became more diffuse. By the blastocyst outgrowth stage, CD44 expression was absent on primary trophoblast giant cells (Fig 2d) and ICM (data not shown).
Fig 4 shows the calculated total cell surface area exposed to labelling antibody at each stage from oocyte to morula. The only significant change in total exposed cell surface area occurred between 2 and 8-cell stages (P < 0.05), with compaction subsequently limiting this area to the outer surface of the embryo. Correspondingly, estimated fluorescence per unit cell surface area, based on these calculations, is shown in Figs 2a, b, c and d on the Y2 axes. From these it can be seen that the pattern of changing kinetics of CAM expression closely followed that of total fluorescence in pre-blastocyst embryos. The increase in total exposed cell surface area at the 8-cell uncompacted stage produced no statistical difference in CAM/unit cell surface area values for any of the molecules.
Line graph showing the change in calculated exposed cell surface area with development from oocyte to 1-cell, 2-cell, uncompacted (u/c) and compacted (c) 8-cell embryos and morula. The ordinate represents total calculated cell surface area accessible to antibody labelling at each stage. Each value represents the average of diameter measurements from at least 4 embryos±standard error (see Materials and Methods).
In general, the antigens investigated were undetectable on zonae pellucidae at all pre-implantation embryo stages. However, we observed brightly staining spots on the zona pellucida at oocyte and 1-cell stages in both NCAM and CD44-labelled, but not other groups (Figs 1 (panel G) and 3a (panel E), respectively). These bright spots were fewer at the 1-cell than oocyte stage.
DISCUSSION
In this study, the expression of CAMs was investigated using immunofluorescent labelling and epifluorescence microscopy in conjunction with quantitative evaluation by photometer and qualitative observation. Large numbers of outbred mouse embryos were used, each at various defined developmental stages and each selected based on optimal morphological and culture criteria. It is clear from the photomicrographs presented that some CAMs may be more highly expressed in particular areas of the cell surface. These may represent as yet undefined functional cellular interactions and/or cytoskeletal re-arrangements. Since only average fluorescence can be determined by this technique, similar to flow cytometry, the actual molecular concentrations at these sites will need to be determined by careful confocal or electron microscopic study.
IgSF molecule expression, ICAM-1 and NCAM, was highest at the oocyte stage of development and decreased with time to consistently undetectable levels by the blastocyst stage and thereafter. With such high levels of ICAM-1, it might be expected that LFA-1 would be correspondingly highly expressed. However, CD11a, the unique a chain of LFA-1, which is the receptor for the cellular ligand, ICAM-117, was undetectable at any of the stages assayed. On the other hand, CD49d, the a chain of the VLA-4, which is the integrin receptor for the cellular ligand, VCAM-1, was clearly expressed, although there was a complete absence of VCAM-1 throughout this period. Like the IgSF molecules, CD49d expression was highest at the earliest stage of development. After an initial reduction in expression, expression again increased during 8-cell and compaction stages and decreased thereafter. In contrast to ICAM-1 and NCAM, CD49d expression was maintained during hatching. Lastly, CD44 showed bimodal kinetics. After a reduction from its highest expression at the oocyte stage, expression increased significantly during compaction and morula stages. At blastocyst hatching expression on blastocyst was restricted to the area adjacent to the operculum in the zona pellucida, but became undetectable after outgrowth in vitro.
Of the three members of the IgSF, investigated here, to our knowledge, only NCAM has previously been canvassed in a semi-quantitative manner in the murine preimplantation embryo18. Although all three molecules have previously been qualitatively reported by Campbell et al., on human preimplantation embryos9, we found expression of VCAM-1 was consistently undetectable in all preimplantation mouse embryos. The reasons for this discrepancy are unclear but certainly may include species differences.
Similarly, in contrast to our study, expression of NCAM was detected on trophectoderm of murine blastocysts, as well as trophoblast and ICM of blastocyst outgrowths in vitro by Kimber et al19. It is unlikely that this difference is due to differential expression and detection of different splice variants between studies, since the antibody used in our study detects all 3 reported NCAM splice variants. Antibody affinity or mouse strain differences between studies may thus be possible explanations for this discrepancy.
The creation of gene knockout animals have shown that ICAM-1, NCAM and VCAM-1 expression are not absolutely required for blastocyst formation and implantation, although they are clearly important in later development18, 20, 21, 22, 23, 24, 25. On the other hand, since ICAM-1 and VCAM-1 mediate and augment critical cell-cell adhesion in the immune response3, absence or significant downregulation of expression of these molecules on invading trophectoderm of the blastocyst may be important in establishing maternal tolerance of the foetal allograft.
Several integrin subunits are expressed on human and/or murine preimplantation embryos7, 8. Their function in embryonic development is not fully understood, but some, such as the α5 chain, are clearly individually important after implantation26, 27. CD49d is the α4 integrin subunit of VLA-4 (a4/b1 integrin) which binds both fibronectin and VCAM-128, 29, 30. The detection of CD49d on murine embryos during pre and peri-implantation stages has not been previously reported, although b1 integrin subunits have been detected, presumptively as the common subunit of other fibronectin receptors, α3/β1 and α5/β1 integrins on the embryo9, 31. Since presence of fibronectin in the capacitated sperm head is associated with successful sperm-egg interaction32, VLA-4 may therefore represent another integrin which contributes to success of fertilisation.
Expression of CD49d on blastocyst at the time of implantation is also of some interest. Although neither fibronectin27, 33 nor VCAM-118 is required for implantation, CD49d expression on trophoblast might be able to make use of VCAM-1, were it cyclically expressed in mouse as it is in human maternal decidua10. Such interaction may make use of ezrin/radixin/moesin (ERM) proteins crosslinking actin filaments with plasma membranes34. The resulting signal transduction could facilitate trophectoderm invasion and further embryonic development. It follows that upregulation of IgSF molecules during local inflammation might retard the tubal passage of the oocyte leading to ectopic implantation.
CD44 is a broadly distributed cell surface protein mediating attachment to extracellular matrix or specific cell surface ligands35. It is the principal cell surface receptor for hyaluronate35, 36 which is widely distributed in extracellular matrices and present within the extracellular matrix of the cumulus cell-oocyte complex37. While successful implantation and embryonic development can occur in its absence38, notwithstanding, it is implicated in a number of biological phenomena, including cell-cell adhesion, migration, embryonic development and pathogenesis39, 40. CD44 is thought to play roles in cell motility and maintenance of cell shape, and is also involved in transformation and metastasis through ERM proteins41. Campbell et al11 reported qualitative CD44 expression on human pre-implantation embryos. Interestingly, we consistently found murine oocytes to express the highest levels of CD44, suggesting its possible involvement in maintaining the oocyte-cumulus-cell complex. Moreover, we also observed specifically labelled brightly staining spots, presumably remnants of cumulus cell matrix, unevenly distributed on zona pellucida in groups of CD44 and NCAM-labelled embryos at oocyte and zygote stages.
Subsequent kinetics of CD44 expression suggest a functional association of this molecule with escape of the blastocyst from zona pellucida at this developmental stage. Which isoforms of CD44 are expressed and whether these are differentially modulated at these stages remain to be determined, since isoform-specific antibodies were not available for this study.
The ICM in both fresh and hatched blastocysts were negative for all adhesion molecules tested, presumably because trophectoderm tight cell junctions prevented their penetration into the blastocyst cavity. To more certainly determine CAM expression on ICM at these stages therefore, it will be necessary to dissociate cell junctions in trophectoderm and in ICM itself or conduct immunohistochemical studies on frozen tissue.
In conclusion, although none of the molecules examined in this study appears individually to be essential for early embryonic development or implantation, expression of these molecules varied significantly and independently during this time. We would speculate that simultaneous differential expression of various CAMs may thus contribute to the fine modulation of intercellular interactions rather than being sine qua non to these adhesive processes, and that their dynamic change may precede changes in activation states39, 42 important for continuing embryonic development and survival. The use of incremental CAM gene knockout animals may help to define these. Although descriptive, therefore, this work lays down a template for the normal expression of these molecules during the pre-implantation and early post-implantation period of the mouse model, when the embryo is probably most at risk for failure. As previously implied12, such novel information may be useful in defining, investigating and subsequently understanding situations in which aberrant expression occurs. These may include genetically predisposed, or environmentally-mediated embryonic failure, from insults such as infection by pathogenic organisms. Our current work is directed towards investigating the putative role of these molecules in the maternal and embryonic responses to virus infection during this early period of embryonic development.
References
Turner ML . Cell adhesion molecules: A unifying approach to topographic biology. Biol Rev 1992; 67:359–77.
Hynes RO, Lander AD . Contact and adhesion specificities in the association, migration, and targeting of cells and axons. Cell 1992; 68:303–22.
Springer TA . Adhesion receptors of the immune system. Nature 1990; 346:425–34.
Damsky C, Sutherland A, Fisher S . Extracellular matrix 5: Adhesive interactions in early mammalian embryogenesis, implantation and placentation. FASEB J 1993; 7:1320–9.
Edelman GM . Expression of cell adhesion molecules during embryogenesis and regeneration. Exp Cell Res 1984; 161:3–16.
Takeichi M . Cadherin cell adhesion receptors as a morphogenetic regulator. Science 1991; 251:1451–5.
Sutherland AE, Calarco PG, Damsky CH . Developmental regulation of integrin expression at the time of implantation in the mouse embryo. Development 1993; 119:1175–86.
Almeida EAC, Huovila APJ, Sutherland AE, et al. Mouse egg integrin alpha-6-beta-1 function as a sperm receptor. Cell 1995; 81:1095–104.
Campbell S, Swann HR, Seif MW, Kimber SJ, Aplin JD . Cell adhesion molecules on the oocyte and preimplantation human embryo. Human Reproduction 1995; 10:1571–78.
Ruck P, Marzusch K, Kaiserling E, et al. Distribution of cell adhesion molecules in decidua of early human pregnancy. Lab Invest 1994; 71:94–101.
Campbell S, Swann HR, Aplin JD, et al. CD44 is expressed throughout pre-implantation human embryo development. Human Reproduction 1995; 10:425–30.
Tian L, Catt JW, O'Neill C, King NJ . Expression of immunoglobulin superfamily cell adhesion molecules on murine embryonic stem cells. Biol. Reprod 1997; 57:561–8.
Quinn P, Kerin JF, Warnes GM . Improved pregnancy rate in human in vitro fertilization with the use of a medium based on the composition of human tubal fluid. Fertility and Sterility 1985; 44:439–98.
Quinn P, Warnes GM, Kerin JF, Kirby C . Culture factors affecting the success rate of in votro fertilization and embryo transfer. Ann NY Acad Sci 1985; 442:195–9.
Conover JC, Gwakin RBL . Pre-loading of mouse oocytes with DNA-specific fluorochrome (hoechst 33342) permits rapid detection of sperm-oocyte fusion. J Reprod Fert 1988; 82:681–90.
Johnson GD . Fading of immunofluorescence during microscopy. A study of the phenomenon and its remedy. J Immunol Meth 1982; 55:231–42.
Marlin SD, Springer TA . Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell 1987; 51:813–9.
Sligh JEJ, Ballantyne CM, Rich SS, et al. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule-1. Proc Natl Acad Sci USA 1993; 90:8529–33.
Kimber SJ, Bentley J, Ciemerych M, Moller CJ, Bock E . Expression of ncam in fertilized pre- and peri-implantation and parthenogenetically activated mouse embryos. Eur J Cell Biol 1994; 63:102–13.
Xu H, Gonzalo JA, Pierre YS, et al. Leukocytosis and resistance to septic shock in intercellular adhesion molecule 1-deficient mice. J Exp Med 1994; 180:95–109.
Cremer H, Lange R, Christoph A, et al. Inactivation of the ncam gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature 1994; 367:455–9.
Gurtner GC, Davis V, Li H, et al. Targeted disruption of the murine vcam1 gene: Essential role of VCAM-1 in chorioallantoic fusion and placentatation. Genes & Development 1995; 9:1–14.
Baldwin TJ, Fazeli MS, Doherty P, Walsh FS . Elucidation of the molecular actions of ncam and structurally related cell adhesion molecules. J Cell Biochem 1996; 61:502–13.
Bowen JA, Hunt JS . Expression of cell adhesion molecules in murine placentas and a placental cell line. Biol Reprod 1999; 60:428–34.
Downs KM . Early placental ontogeny in the mouse. Placenta 2002; 23:116–31.
Yang JT, Rayburn H, Hynes RO . Embryonic mesodermal defects in alpha-5 integrin-deficient mice. Cell 1993; 119:1093–105.
Watt FM, Hodivala KJ . Fibronectin and integrin knockouts come unstuck. Curr Biol 1994; 4:270–72.
Guan J, Hynes RO . Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor alpha-4-beta-1. Cell 1990; 60:53–61.
Hynes RO . Integrins: Versatility, modulation, and signaling in cell adhesion. Cell 1992; 69:11–25.
Berlin C, Berg EL, Briskin MJ, et al. Alpha-4-beta-7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 1993; 74:185–95.
Evans JP, Schultz RM, Kopf GS . Mouse sperm-egg plasma membrane interactions: Analysis of roles of egg integrins and the mouse sperm homologue of PH-30 (fertilin) beta. J Cell Sci 1995; 108:3267–78.
Hoshi K, Sato A, Sasaki H, Tsuiki A, Yanagida K . Localization of fibronectin on the surface of human spermatozoa and relation to the sperm-egg interaction. Fertility and Sterility 1994; 61:542–7.
George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO . Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development 1993; 119:1079–91.
Barreiro O, Yanez-Mo M, Serrador JM, et al. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol 2002; 157:1233–45.
Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B . CD44 is the principal cell surface receptor for hyaluronate. Cell 1990; 61:1303–13.
Miyake K, Underhill CB, Lesley J, Kincade PW . Hyaluronate can function as a cell adhesion molecule and CD44 participates in hyaluronate recognition. J Exp Med 1990; 172:69–75.
Dandekar P, Aggeler J, Talbot P . Structure, distribution and composition of the extracellular matrix of human oocytes and cumulus masses. Human Reproduction 1992; 7:391–8.
Schmits R, Filmus J, Gerwin N, et al. CD44 regulates hematopoietic progenitor distribution, granuloma formation, and tumorigenicity. Blood 1997; 90:2217–33.
Laurent TC, Fraser JR . The properties and turnover of hyaluronan. Ciba Foundation Symposium 1986; 124:9–29.
Fillit HM, Blake M, MacDonald C, McCarty M . Immunogenicity of liposome-bound hyaluronate in mice. J Exp Med 1988; 168:971–82.
Tsukita S, Yonemura S, Tsukita S . ERM proteins: Head-to-tail regulation of actin-plasma membrane interaction. TIBS 1997; 22:53–58.
Lesley J, Hyman R, Kincade PW . CD44 and its interaction with extracellular matrix. Adv Immunol 1993; 54:271–335.
Acknowledgements
This work was supported by grant No. 950175 from the Australian National Health and Medical Research Council. D.P. Lu and L. Tian each made an equivalent contribution to this publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
LU, D., TIAN, L., O' NEILL, C. et al. Regulation of cellular adhesion molecule expression in murine oocytes, peri-implantation and post-implantation embryos. Cell Res 12, 373–383 (2002). https://doi.org/10.1038/sj.cr.7290139
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.cr.7290139
Keywords
This article is cited by
-
Hashimoto’s thyroiditis impairs embryo implantation by compromising endometrial morphology and receptivity markers in euthyroid mice
Reproductive Biology and Endocrinology (2019)
-
Biochemical alterations in the oocyte in support of early embryonic development
Cellular and Molecular Life Sciences (2017)
-
EpCAM is decreased but is still present in uterine epithelial cells during early pregnancy in the rat: potential mechanism for maintenance of mucosal integrity during implantation
Cell and Tissue Research (2015)