Abstract
OBJECTIVE:
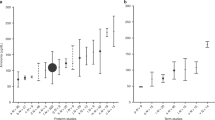
Currently blood urea nitrogen (BUN) is commonly used as a marker of protein intolerance in very preterm infants. The purpose of this study was to evaluate the relationship between amino-acid intakes and BUN concentrations during the early neonatal period in preterm neonates.
STUDY DESIGN:
Retrospective review of BUN concentration data from 121 infants with birthweight ≤1250 g receiving exclusive parenteral nutrition over the first 72 hours of life.
RESULTS:
There were 136 separate BUN concentration values. Amino-acid intake range was 0 to 3.7 g kg−1 day−1 and nonprotein calorie intake range was 15 to 45 kcal kg−1 d−1. There was no correlation between BUN concentration and amino-acid intake (p=0.2 and r2=0.01).
CONCLUSIONS:
In parenterally nourished preterm neonates amino-acid intake is not correlated with BUN concentration in the first days of life. Therefore, limiting amino-acid intake based on BUN concentration is not warranted in this patient population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Johnson JD, Albritton WL, Sunshine P . Hyperammonemia accompanying parenteral nutrition in newborn infants. J Pediatr 1972;81 (1):154–161.
American Academy of Pediatrics. Committee on Nutrition. In: Kleinman RE, editor. Pediatric Nutrition Handbook. 4th ed. Elk Grove Village, IL: American Academy of Pediatrics; 1998.
Thureen PJ, Melara D, Fennessey PV, Hay Jr WW . Effect of low versus high intravenous amino-acid intake on very low birthweight infants in the early neonatal period. Pediatr Res 2003;53 (1):24–32.
Kashyap S, Heird W . Protein requirements of low birthweight, very low birthweight, and small for gestational age infants. In: Raiha N, editor. Protein Metabolism During Infancy. New York: Raven Press; 1993. p. 133–151.
Rubecz I, Mestyan J, Varga P, Klujber L . Energy metabolism, substrate utilization, and nitrogen balance in parenterally fed postoperative neonates and infants. The effect of glucose, glucose+amino-acids, lipid+amino-acids infused in isocaloric amounts. J Pediatr 1981;98 (1):42–46.
Zlotkin SH, Bryan MH, Anderson GH . Intravenous nitrogen and energy intakes required to duplicate in utero nitrogen accretion in prematurely born human infants. J Pediatr 1981;99 (1):115–120.
Duffy B, Gunn T, Collinge J, Pencharz P . The effect of varying protein quality and energy intake on the nitrogen metabolism of parenterally fed very low birthweight (less than 1600 g) infants. Pediatr Res 1981;15 (7):1040–1044.
van Toledo-Eppinga L, Kalhan SC, Kulik W, Jakobs C, Lafeber HN . Relative kinetics of phenylalanine and leucine in low birthweight infants during nutrient administration. Pediatr Res 1996;40 (1):41–46.
Anderson TL, Muttart CR, Bieber MA, Nicholson JF, Heird WC . A controlled trial of glucose versus glucose and amino-acids in premature infants. J Pediatr 1979;94 (6):947–951.
Yu VY, James B, Hendry P, MacMahon RA . Total parenteral nutrition in very low birthweight infants: a controlled trial. Arch Dis Child 1979;54 (9):653–661.
Saini J, MacMahon P, Morgan JB, Kovar IZ . Early parenteral feeding of amino-acids. Arch Dis Child 1989;64(10 Spec No.):1362–1366.
van Lingen RA, van Goudoever JB, Luijendijk IH, Wattimena JL, Sauer PJ . Effects of early amino-acid administration during total parenteral nutrition on protein metabolism in pre-term infants. Clin Sci 1992;82 (2):199–203.
van Goudoever JB, Colen T, Wattimena JL, Huijmans JG, Carnielli VP, Sauer PJ . Immediate commencement of amino acid supplementation in preterm infants: effect on serum amino acid concentrations and protein kinetics on the first day of life. J Pediatr 1995;127 (3):458–465.
Thureen PJ, Anderson AH, Baron KA, Melara DL, Hay Jr WW, Fennessey PV . Protein balance in the first week of life in ventilated neonates receiving parenteral nutrition. Am J Clin Nutr 1998;68 (5):1128–1135.
Rivera Jr A, Bell EF, Bier DM . Effect of intravenous amino acids on protein metabolism of preterm infants during the first three days of life. Pediatr Res 1993;33 (2):106–111.
Denne SC, Karn CA, Ahlrichs JA, Dorotheo AR, Wang J, Liechty EA . Proteolysis and phenylalanine hydroxylation in response to parenteral nutrition in extremely premature and normal newborns. J Clin Invest 1996;97 (3):746–754.
Ziegler EE . Protein requirements of preterm infants. In: Fomon SJ, Heird WC, editors. Energy and Protein Needs During Infancy. New York: Academic Press; 1986.
Ziegler EE . Protein in premature feeding. Nutrition 1994;10 (1):69–71.
Eaton BM, Yudilevich DL . Uptake and asymmetric efflux of amino acids at maternal and fetal sides of placenta. Am J Physiol 1981;241 (3):C106–C112.
Lemons JA, Adcock 3rd EW, Jones Jr MD, Naughton MA, Meschia G, Battaglia FC . Umbilical uptake of amino-acids in the unstressed fetal lamb. J Clin Invest 1976;58 (6):1428–1434.
Battaglia FC, Meschia G . Fetal nutrition. Annu Rev Nutr 1988;8:43–61.
Battaglia FC, Meschia G . An Introduction to Fetal Physiology. Orlando: Academic Press; 1986.
Gresham EL, Simons PS, Battaglia FC . Maternal–fetal urea concentration difference in man: metabolic significance. J Pediatr 1971;79 (5):809–811.
Acknowledgements
This work was supported by National Institutes of Health Grants RO3HD39842 and M01RR00069, General Clinical Research Center Program, National Centers for Research Resources.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ridout, E., Melara, D., Rottinghaus, S. et al. Blood Urea Nitrogen Concentration as a Marker of Amino-Acid Intolerance in Neonates with Birthweight Less than 1250 g. J Perinatol 25, 130–133 (2005). https://doi.org/10.1038/sj.jp.7211215
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jp.7211215
This article is cited by
-
Assessment of catabolic state in infants with the use of urinary titin N-fragment
Pediatric Research (2022)
-
Effect of increased enteral protein intake on plasma and urinary urea concentrations in preterm infants born at < 32 weeks gestation and < 1500 g birth weight enrolled in a randomized controlled trial – a secondary analysis
BMC Pediatrics (2018)
-
Bone status in preterm infant: influences of different nutritional regimens and possible markers of bone disease
Journal of Perinatology (2016)
-
Impact of renal function and protein intake on blood urea nitrogen in preterm infants in the first 3 weeks of life
Journal of Perinatology (2015)
-
Standardised neonatal parenteral nutrition formulations – an Australasian group consensus 2012
BMC Pediatrics (2014)