Abstract
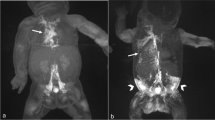
We report a neonate with severe congenital chylothorax. Subcutaneous octreotide was added to the standard treatment regime. The chylothorax resolved with no observed side effects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Van Straaten HLM, Gerards LJ, Krediet TG . Chylothorax in the neonatal period. Eur J Pediatr 1993;152:2–5.
Van Aerde J, Campbell AN, Smyth JA, Lloyd D, Bryan MH . Spontaneous chylothorax in newborns. Am J Dis Child 1984;138:961–964.
Dubin PJ, Kind IN, Gallagher PG . Congenital chylothorax. Curr Opin Pediatr 2000;12:505–509.
Ryckman F, Rosenkrantz J . Thoracic surgical problems in infancy and childhood. Surg Clin North Am 1985;65:1423–1454.
Kugelman A, Gonen R, Bader D . Potential role of high-frequency ventilation in the treatment of severe congenital pleural effusion. Pediatr Pulmonol 2000;29:404–408.
Levine C . Primary disorders of the lymphatic vessels — a unified concept. J Pediatr Surg 1989;24:233–240.
Engum SA, Rescoria FJ, West KW, Scherer LR, Frosfeld JL . The use of pleuroperitoneal shunts in the management of persistent chylothorax in infants. J Pediat Surg 1999;34:286–290.
Alvarez JR, Kalache KD, Grauel EL . Management of spontaneous congenital chylothorax: oral medium chain triglycerides versus total parenteral nutrition. Am J of Perinatol 1999;16:415–420.
Al-Tawil K, Ahmed G, Al-Hathal M, Al-Jarallah Y, Campbell N . Congenital chylothorax. Am J Perinatol 2000;17:121–126.
Selle JG, Snyder III WH, Schreiber JT . Chylothorax: indications for surgery. Ann Surg 1973;177:245–249.
Taketomo C, Hodding J, Kraus D . Pediatric Dosage Handbook. Hudson: Lexi-Comp Inc.; 1998. p. 800–803.
Stanley C . Hyperinsulinism in infants and children. Pediatr Clin North Am 1997;44:363–374.
Pratap U, Slavik Z, Ofoe V, Onuzo O, Franklin R . Octreotide to treat post-operative chylothorax after cardiac operations in children. Ann Thorac Surg 2001;72:1740–1742.
Cheung Y, Leung M, Yip M . Octreotide for treatment of postoperative chylothorax. J Pediatr 2001;139:157–159.
Demos NJ, Teresi A . Congenital lung formations: a unified concept and a case report. J Thorac Cardiovasc Surg 1975;70:260–264.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Young, S., Dalgleish, S., Eccleston, A. et al. Severe Congenital Chylothorax Treated With Octreotide. J Perinatol 24, 200–202 (2004). https://doi.org/10.1038/sj.jp.7211053
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jp.7211053
This article is cited by
-
Octreotide Use in Neonates: A Case Series
Drugs in R&D (2018)
-
Congenital Chylothorax - Successful management with chemical pleurodesis
The Indian Journal of Pediatrics (2010)
-
Somatostatin or octreotide as treatment options for chylothorax in young children: a systematic review
Intensive Care Medicine (2006)