Abstract
Many populations of the buprestid leaf-mining beetle, Brachys tessellatus, from central South Carolina, USA, show highly skewed sex ratios, ranging from 1.3 to 6.0 females per male. We have identified a Rickettsia bacterium that is associated with sex ratio distortion (SRD) and selective killing of male embryos in B. tessellatus. Molecular assays of infection by this bacterium are highly associated with SRD within families, and treatment with an antibiotic (tetracycline) increases the number of male eggs that hatch and develop. The 16S rDNA sequence indicates that this is a novel Rickettsia, most closely related to Rickettsia bellii (a tick-associated bacterium) and a pea-aphid Rickettsia. It is also related to a Rickettsial bacterium that causes male-killing in an unrelated ladybird beetle species. Low levels of parthenogenesis are also observed in this system (about 10% of females) and may be the result of selection due to male rarity, or a direct result of infection by the Rickettsia.
Similar content being viewed by others
Introduction
Inherited microorganisms are associated with a variety of reproductive alterations in their hosts, including cytoplasmic incompatibility, thelytokous parthenogenesis, feminization of genetic males, and male-killing (reviewed in Werren & O’Neill, 1997). These alterations are generally adaptive for the microorganisms because they are typically inherited maternally through the egg cytoplasm, but are not transmitted via sperm. As a result, inherited microbes often have an asymmetrical inheritance pattern with high maternal (female) inheritance and low or absent paternal (male) inheritance. Selection therefore favours such microorganisms to bias sex ratio or sex determination towards females, the transmitting sex (Werren & O’Neill, 1997).
Male-killing microorganisms are widespread in nature (see Hurst et al., 1997 for a review), and involve a wide range of microbial taxa and eukaryotic hosts. Examples include gamma proteobacteria such as Arsenophonus nasoniae, the male-killing bacterium of Nasonia (Gherna et al., 1991), alpha proteobacteria such as Rickettsia in ladybeetles (Werren et al., 1995b) and Wolbachia in Acraea butterflies (Hurst et al., 1999), flavobacteria in beetles (Hurst et al., 1997), spiroplasms in Drosophila (Williamson et al., 1999) and microsporidia (protozoa) in mosquitoes (Hurst, 1991) and amphipods (Dunn & Hatcher, 1997). The pattern suggests that male-killing by resident inherited microbes can readily evolve whenever the ecological circumstances are appropriate. Male-killing can be selectively favoured under two general circumstances: (a) when lethality of males increases the fitness of infected females in the family or (b) when male-killing provides an inoculum of microbes for horizontal (infectious) transmission. Although there is some evidence that male-killing may provide an inoculum for horizontal transmission in some systems (Hurst, 1991), a fitness advantage to infected female siblings is believed to be the primary advantage for male-killing (Werren, 1987; Hurst et al., 1997). Possible advantages include reduced inbreeding, reduced sibling competition for resources and additional food provided by egg cannibalism. It should be noted though, that these are advantages to the male-killing microbes but not to the host, which suffers a major fitness decrement from male-killing. Specifically, for male-killing to be advantageous for a heritable microbe, the advantage of killing males must only slightly increase the fitness of infected female siblings; whereas for a male-killing nuclear gene to be selectively favoured, the fitness of female siblings must increase at least two-fold (Werren, 1987).
The biology of several species with male-killing microbes is consistent with these ideas. For example, egg cannibalism occurs in the ladybird beetle Adalia bipunctata and has been shown to increase survival of newly hatched larvae. This species has several male-killing microbes (Hurst & Jiggins, 2000). Similarly, sibling larval competition is expected within the hosts of Nasonia parasitoid wasps, another species with male-killing microorganisms. However, the selective advantage of male-killing has not been definitively demonstrated in any system (e.g. see Balas et al., 1996).
Here we describe the discovery of a new Rickettsia associated with male-killing in the buprestid leaf-mining beetle Brachys tessellatus (F.). B. tessellatus is a univoltine leaf-mining beetle found in the south-eastern United States, primarily within the sandhills ecosystem of South Carolina, North Carolina, and Georgia (Turnbow & Franklin, 1981). The adult portion of its life cycle is spent feeding and ovipositing on several species of oak. Larvae mine (i.e. eat) the inner mesophyll of oak leaves. This system has several interesting features that make it suitable for field studies of the evolution and consequences of male-killing bacteria, including highly skewed sex ratios in nature (1.3:1 to 6:1 females to males; see Fig. 1), indicating achievement of appreciable frequencies of the sex ratio distorter, and parthenogenetic reproduction at low levels (up to 10%), a possible selective consequence of scarcity of males. In this paper we present the results of field surveys of sex ratio variation among populations, an experimental antibiotic treatment to test for microbial effects on sex ratio distortion (SRD), and molecular analysis to identify the specific microbe associated with male-killing and SRD in this system. In addition, tests were conducted to determine the incidence of facultative parthenogenesis in B. tessellatus.
Materials and methods
Basic field studies
Preliminary field studies were initiated in 1996 to assess sex ratio variation in B. tessellatus and a closely related congener, B. ovatus. Beetle larvae were collected from a total of 18 sites within a 20-km radius of Columbia, SC, during October and November. Between 26 and 2897 larvae were collected from each site for B. tessellatus (total for all sites = 7093). These larvae were brought to the laboratory and incubated at 8°C for 2–3 months, followed by gradual warming to 28°C (1°C steps per 2–3 days) at which time all living larvae had completed pupation and final ecdysis. Following the completion of development all beetles were sexed by visual inspection of their abdomens. This method provides for unambiguous gender identification (Turnbow & Franklin, 1981; Waddell & Mousseau, 1996).
Tests for parthenogenesis
During the spring of 1997 an experiment was designed to test for the ability of virgin females to produce viable (presumably parthenogenetic) eggs. Wild-caught larvae were collected from the field during the fall of 1996 at Sesquicentennial State Park in Columbia, SC. Following extraction from leaves, these larvae were maintained singly in cotton-stoppered 12 × 50 mm glass tubes to assure virginity of emerging adults. Following final ecdysis in May 1997, the individual virgin females were enclosed in Agryl Agrocloth polypropylene mesh bags (25 × 30 cm) and placed on clusters of 5–8 leaves of Quercus laevis (turkey oak), their preferred host plant (Turnbow & Franklin, 1981; Waddell & Mousseau, 1996). These bags allowed airflow and photosynthesis to occur, but effectively excluded wild beetles from entering or experimental beetles from escaping. Virgin female beetles were left on leaves for approximately 2–3 weeks at which time most females had laid some unfertilized eggs. Following the production of these first eggs, females were rotated at two or three day intervals to new leaf clusters. Prior to use, all leaves were scrutinized for the presence of wild eggs which if found, were removed from the leaf. One reason this site was chosen was for its relatively low numbers of wild beetles so as to reduce the chances of wild (fertilized) eggs that could interfere with the experiment. Unfertilized eggs were protected from predation within Agryl bags, which also served to keep wild females from laying eggs on the leaves. Two to four weeks following egg lay, leaves were inspected for the presence of larval initiates (i.e. newly hatched larvae). Larval initiates were easily identified by a characteristic brown area surrounding the hatched egg on the leaf, and this assay was verified by microscopic examination of larvae within mines. Eggs showing signs of development were scored as parthenogens. The expression of parthenogenesis was assessed as both the percentage of total eggs laid per female, and the frequency of virgin females showing viable unfertilized eggs.
Antibiotic treatment experiments
During the summer of 1997, an experiment was initiated to determine whether antibiotic (tetracycline) treatment affected the level of mortality and sex ratio among offspring of mated pairs. Following the tests for parthenogenesis (above), these same females were divided into two groups (treatment and control), paired with virgin males, enclosed in Agryl mesh bags, and tied onto clusters of 5–8 turkey oak leaves. Following an initial nuptial period of 5–7 days, mating pairs were rotated to new leaf clusters on a 2-day rotation schedule. Eggs produced during the nuptial period were not included in subsequent analyses. Upon removal of the pair from a leaf cluster, cluster identification labels were applied to the leaves and the number of eggs laid were counted and their location marked with permanent-ink pens. Leaf clusters containing eggs were covered with an Agryl mesh bag to protect the eggs from predation and to allow for collection of the experimental larvae when the leaves abscised from the trees in fall of 1998. Egg viability was assessed approximately two weeks after oviposition. Larvae were counted and the protective bags replaced on the leaf cluster. The treatment group was comprised of approximately 100 wild-caught B. tessellatus females that were also subjected to the test for parthenogenesis, then paired with virgin males, and tied onto leaf clusters of Q. laevis. These pairs were maintained under the same procedure as the control group, except that they were fed tetracycline prior to and during oviposition. Tetracycline is a broad spectrum antibiotic known to purge insects of several different endosymbionts (Gomi et al., 1997; Hoerauf et al., 2000). Tetracycline was applied on the leaf clusters at a concentration of 2500 mg/L water. The tetracycline solution was sprayed on the leaves and allowed to dry before mating pairs were tied onto the clusters in the cotton mesh bags. Mating pairs were allowed to eat and oviposit on the leaves on a 2-day rotation schedule as described for the control group. Eggs were counted, marked and viability assessed as with the control group.
A second control was conducted using the congeneric beetle species, B. ovatus, which past surveys suggested did not show sex ratio distortion. This species has a very similar life history except that it is about 50% smaller by weight and prefers to oviposit and feed on a closely related host, the willow oak, Quercus incana. Populations of B. ovatus that were studied in 1996 and prior years maintained a consistent sex ratio of approximately 1:1. This made B. ovatus an appropriate control group organism to compare with the treated and untreated B. tessellatus groups. Approximately 25 pairs of B. ovatus were rotated along with the untreated group of B.tessellatus and assayed in an identical fashion to the other control pairs.
We collected the offspring from all females in November of 1997 and over-wintered them in the laboratory on a temperature schedule of 8°C for 3 months, followed by a gradual warming to 28°C in 1°C increments every 2–3 days. Adult offspring emerged in the laboratory in May of 1998 and were sexed and weighed, and survivorship to adulthood was assessed across the treatment groups.
Molecular identification of associated bacterium
Samples of preserved (95% ethanol) B. tessellatus adults from populations showing highly distorted and less distorted sex ratios were initially screened for presence of Wolbachia bacteria using 16S rDNA Wolbachia-specific primers (Werren & Windsor, 2000). However, Wolbachia were not detected. Efforts were then initiated to determine whether the beetles harboured other symbiotic bacteria, following modifications of previously published procedures (Werren et al., 1994). DNA was extracted from dissected ovaries of individual beetles using the QIAamp tissue kit. Special care was taken to avoid contamination with bacterial DNA during extraction procedures (see Werren et al., 1995a). General prokaryotic 16S rDNA primers (fD1 and rP2, Weisburg et al., 1991) were then used to amplify the bacterial gene from samples. An intense band of expected size was amplified from some samples. Under the assumption that the amplified band represented a nearly pure sample from a single bacterial species, direct sequencing of the band was performed. If the DNA was from a heterologous population of different bacteria, then direct sequencing would fail to produce a reliably scored sequence.
The QIAquick PCR purification kit was used to repurify DNA from the PCR reaction, and then direct sequencing was performed using an ABI Prism 377 automated sequencer. Sequences were obtained from products of two different beetles; these proved to be identical except for minor sequencing artifacts that were resolved by examination of the sequencing reaction profiles. Reliable sequence was obtained for 735 bases of the 5′ end of the 16S rDNA gene.
The sequence was then aligned to a diverse array of known 16S rDNAs, which confirmed that the bacterium was a close relative of intracellular bacteria in the genus Rickettsia. For phylogenetic comparison, sequences from 10 Rickettsia (including the male-killing Rickettsia previously identified from ladybird beetles, Werren et al., 1994), Ehrlichia equii and Anaplasma marginale were used. Ehrlichia and Anaplasma are sister genera of Rickettsia and served as outgroups for the comparison. The program PAUP (version 4.0b#a) was employed for the analysis. Sequences were evaluated using the neighbour-joining method of similarity data corrected by the Jukes–Cantor algorithm. A heuristic search was performed, and the observed tree was then evaluated by 100 bootstrap comparisons.
Association of Rickettsia with sex ratio distortion
Based on the identification of a Rickettsia present in B. tessellatus, a second field study was conducted to determine whether the bacterium was associated with distorted sex ratios in field populations. We collected beetles from a site near the Clemson Sandhills Research and Education Center in Columbia, South Carolina in spring of 1998. Approximately 200 male–female pairings were performed and placed on Q. laevis trees at two locations: Sesquicentennial State Park in Columbia, South Carolina, and the Savannah River Site near Aiken, SC. Mated pairs were maintained in the field as in expt. 2. Beetles were allowed to reproduce over the summer months, and offspring were collected in November of 1998. Offspring were maintained in the laboratory until they emerged as adults at which time they were sexed and preserved in 100% ethanol in Eppendorf tubes at −70°C for PCR analysis of bacterial presence in the different families.
Offspring were assayed for the Rickettsia using modifications of previously published methods (Werren et al., 1994, 1995b). One female offspring from each family was screened for the Rickettsia. Whole abdomens were dissected from preserved beetles and then rinsed in sterile water prior to extraction of DNA using the Chelex resin. Uninfected Nasonia wasps were also processed to serve as extraction controls. The DNA templates were screened using specific primers designed from the 16S rDNA sequence from the B. tessellatus Rickettsia (Ric F (5′–3′): CCC ATC AGT ACG GAA TAA CT, Rif R (5′–3′): GAA TTC CAT CAT CCC CTA CTA CAC). These amplify a fragment of 506 bp from the 5′ prime portion of the 16S gene. A sample of 2 μL of Chelex template per 100 μL reaction was used with the following amplification protocol (Predwell: 95°C for 2 min, 35 cycles at 95°C for 30 s, one cycle at 57°C for 1 min, one cycle at 72°C for 45 s, and Postdwell at 72°C for 5 min). A 1% agarose gel and ethidium bromide staining were used to detect PCR products following electrophoresis. Negative samples were checked with general eukaryotic rDNA primers to confirm amplifiability of the DNA extraction, as described in Werren et al. (1995b). Any samples failing to amplify this product were excluded from the analysis.
Results
Sex ratios in field populations
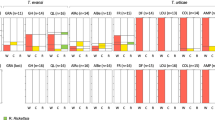
Field surveys revealed highly distorted sex ratios in some populations of B. tessellatus. Population sex ratios ranged from 1.3 to 6 females per male in the 18 study populations (Fig. 1). Sex ratios for all populations were significantly different from 1:1 (based on Chi-squared tests) and there was significant variation among populations for sex ratio (G-test for independence, G=244, P ≪ 0.001). Population estimates were based upon reasonable sample sizes (between 26 and 2897 individuals; total=7093) and therefore estimates were not likely to be subject to large sampling errors. However, these highly skewed sex ratios could be due to primary sex ratio differences, differential embryonic mortality (e.g. due to a male-killing bacterium), or differential survival to adulthood.
The sex ratio bias found in B. tessellatus populations did not occur in its congener B. ovatus. Field surveys of wild B. ovatus adults in 1996 found sex ratios of 56 females to 54 males, while adults emerging from experimental crosses in 1997 (i.e. pupation occurred in the laboratory) were found to be 173 females to 146 males, which was not significantly different from a 1:1 expectation (1.18 females per male; Chi-squared = 2.29, d.f.=1, P < 0.2).
Finally, preliminary data indicated that B. tessellatus has a much lower hatching rate of eggs than did its sister species, B. ovatus (1996 hatch rates for wild-caught B. ovatus were 58.8% (n=1077) and 31.6% for B. tessellatus (n=7879) (G-test of independence, G=1420, P < 0.001)). Taken together, the skewed adult sex ratios and the low hatching rates of B. tessellatus, suggested that a male-killing bacterium was possibly present at appreciable frequencies in this species. Therefore, attempts to demonstrate such a bacterium were initiated.
Tests for parthenogenesis
Parthenogenetic reproduction was found to occur at a low rate in B. tessellatus. Of the 713 unfertilized eggs produced by experimental females, 87 showed signs of early development, and 43 completed development to adulthood. This suggests that at the population level, as many as 12.2% of the eggs could be the result of parthenogenesis. The incidence of parthenogenesis varied greatly among females. Of the 119 females surveyed, 34 (29%) produced at least one viable egg. Although leaves were screened for the presence of wild eggs prior to their use in this experiment, it is nonetheless possible that a few were not detected and may have inflated our estimates of parthenogenesis. Control surveys indicated that error rates for wild egg detection were about 1% at this site and thus probably did not contribute significantly to our estimate of parthenogenesis.
Antibiotic treatment experiments
Results clearly indicate that a male-killing bacterium sensitive to tetracycline is responsible for the sex ratio distortion found in B. tessellatus. Analysis of offspring from the treatment vs. non-treatment groups showed about twice the hatch rate in treated samples (means ± 1 SE.; treated B. tessellatus 46.2 ± 1.1% hatch rate, untreated B. tessellatus 24.6 ± 0.9% hatch rate, untreated B. ovatus, 46.8 ± 4.2% hatch rate; ANOVA using angular transformation on initiation rates, F=10.1, d.f.=2,773, P < 0.0001). Following antibiotic treatment hatch rates in B. tessellatus increased to levels similar to those found in B. ovatus.
The sex ratio bias of treated B. tessellatus among offspring that survived to adulthood (i.e. secondary sex ratio of the samples) decreased significantly in comparison to that found from untreated B. tessellatus. Tetracycline-treated females produced significantly more male offspring on average than did untreated control females (a total of 103 males and 201 female offspring for the treated group vs. 31 males and 270 females for the untreated control group; G-test for homogeneity, G=52, d.f.=1, P < 0.0001). At the family level, for females producing more than four offspring surviving to adulthood, treated females produced significantly more male offspring on average than did control females (untreated females, mean proportion female offspring using arcsin(sqrt) transformation on family means=0.97, 95% CI=0.93–0.99, N=31 families; treated females=0.74, 95% CI=0.62–0.85, N=34 families; ANOVA, F=17.7, P < 0.0001, Wilcoxon 2-sample test, S=1300, Z=3.84, P < 0.0001), and there was a significant negative correlation between egg hatch rate and family sex ratio distortion (i.e. families with high hatch rates had lower sex ratio distortion; rs=0.15, d.f.=176, P < 0.05). In addition, 67.7% of the untreated families produced broods consisting of all females (21/31), whereas only 26.5% of the treated families produced all-female broods (9/34; G-test for homogeneity, G=11.23, d.f.=1, P < 0.0001).
Therefore, it can be concluded that antibiotic treatment causes both an increase in the proportion of surviving males and an increase in the percentage of eggs hatching, consistent with the view that a male-killing microorganism that acts during embryonic development, is present in the population.
Molecular identification of associated bacterium
Based upon alignment, the B. tessellatus (Btess) bacterium showed high sequence similarity to bacteria in the genus Rickettsia, an alpha proteobacterial group of intracellular parasitic bacteria. Many Rickettsia are disease-causing agents of mammals that are vectored by arthropods (e.g. Dumler, 1997; Myers & Sexton, 1994; Yunker, 1996). Examples include Rickettsia prowazekii (causative agent of Brill–Zinsser disease; Turcinov et al., 2000), R. tsutsugamushi (causative agent of scrub typhus) and Rickettsia rickettsii (causative agent of Rocky Mountain Spotted Fever). Other bacteria in the genus have unknown aetiology in their hosts, and one (the AB bacterium) is associated with male-killing in the ladybird beetle, Adalia bipunctata (Werren et al., 1994).
The close phylogenetic relationship of the B. tessellatus bacterium to Rickettsia is shown in Fig. 2. Approximately 735 bp of sequence were aligned to 12 bacterial sequences (10 Rickettsia, and two sister taxa, Ehrlichia equii and Anaplasma marginale) and evaluated by neighbour-joining using the Jukes–Cantor correction for multiple changes. The Btess bacterium is monophyletic with other Rickettsia, with 100 of 100 bootstrap replicates conforming relative to the two outgroups Ehrlichia equii and Anaplasma marginale. These two genera are currently the nearest known to the genus Rickettsia, based upon 16S rDNA sequences (Weisburg et al., 1989; Roux & Raoult, 1995).
Within Rickettsia, the Btess bacterium shows greatest similarity to a clade containing the AB bacterium, pea-aphid bacterium and R. bellii (90/100 bootstrap comparisons conforming). The AB bacterium causes male-killing in the ladybird beetle, Adalia bipunctata (Werren et al., 1994). A phenotype has not yet been ascribed to the Rickettsia found in the pea aphid (Chen et al., 1996) and R. bellii is a widespread Rickettsia found in ticks, but its phenotypic effects on ticks are not described (Philip et al., 1983). The Btess Rickettsia is closely related to R. bellii (one difference over 735 bases, 0.1% different) and the pea-aphid bacterium (3 base differences over 735 bases, 0.4% different). However, it should be kept in mind that this level of difference in the 16S rDNA could correspond to considerable evolutionary time. Estimates for 16S rDNA evolution range from around 1–2% per 50 million years (Ochman & Wilson, 1987; Moran et al., 1993; Ochman et al., 1999), suggesting that these bacteria diverged 2.5–5 and 10–20 million years ago, respectively.
Association of Rickettsia with sex ratio distortion
Our results clearly establish the presence of a Rickettsia within some individuals of B. tessellatus, and antibiotic treatments indicate that a bacterium is responsible for male-killing in this species. Therefore, studies were undertaken to determine whether the Rickettsia we isolated is associated with male-killing in B. tessellatus.
Beetles were reared on trees in the field in mesh bags to keep track of families. These were then scored for sex ratio and family size, and a sample of beetles were screened for the Btess Rickettsia using rickettsial-specific PCR primers. The analysis showed a strong correlation between the amount of female bias seen in families and the infection rate with the endosymbiont Rickettsia (Table 1). Of 51 families that were tested, 90% of families containing no males were infected, followed by 58% of families containing 1–25% males, 19% of families containing 25–50% males, and 17% of families containing greater than 50% males were infected with the endosymbiont (G-test for homogeneity, G=19.1, d.f.=3, P < 0.001). However, the bacterium was not found in all families showing sex ratio distortion, and was also found in some families that produced males (i.e. male-killing was not 100%). Possible explanations are discussed below.
In the entire sample, 46.3% of families (N=51) were found to be infected with Btess Rickettsia. This is likely to represent an underestimate because (a) small families (less than four offspring) were excluded from the analysis and those containing the male-killing Rickettsia are more likely to have reduced family size and (b) the bacterium may not have 100% transmission to progeny. Nevertheless, this frequency represents among the highest found for a male-killing bacterium in natural populations and is consistent with the strongly skewed population sex ratios observed.
We also screened 3–5 females per family for four all-female families and for four 50%-female families to get an estimate of transmission rate of the bacteria to females within these two classes. Although sample sizes are small, transmission was found to be significantly higher in the 100% families than in the 50% male families; 19 of 20 females in the 100% female families were infected while only 6 of 14 females were infected in the 50% female (i.e. normal SR) families (Fisher exact test, P < 0.002). This result suggests that expression of male-killing and transmission may be correlated, perhaps due to differences in bacterial density in reproductive tissues of females.
Discussion
The combination of comparative, experimental and corroborative data presented in this paper strongly support the hypothesis that the Btess Rickettsia bacterium is the causal agent responsible for the patterns of sex ratio distortion observed within and among populations of the turkey oak leaf-mining beetle, Brachys tessellatus, and that this bacterium causes SRD by selectively killing male embryos before they hatch. This support stems from the following observations. First, antibiotic treatment increases embryonic hatching rates closer to those expected if the Btess bacterium was selectively killing males (i.e. hatching rates are almost doubled, and are very similar to its sympatric congener that does not express SRD). Second, antibiotic treatment increases the proportion of males surviving to adulthood, leading to sex ratios closer to those expected in the absence of a male-killing bacterium. And third, there was a very strong relationship at the family level between infection and SRD (Table 1). Although these data strongly support a bacterial source for male mortality, they do not rule out the possibility that other bacteria or tetracycline sensitive microorganisms are involved. It is possible, for example, that two different endosymbionts might be present and working synchronously to cause sex ratio distortion in this system. Tetracycline treatment would arguably reduce the infection rate of all sensitive microbes present in the organism. However, the screening process used to identify prokaryotes present in samples and the specific correlation of Btess Rickettsia to sex ratio distortion among full-sib families suggests that it alone is causing sex ratio distortion in B. tessellatus. Indeed, only the Btess Rickettsia bacterium was found in any detectable concentration in the samples showing SRD.
This bacterium occurs at among the highest frequency observed for any known male-killing endosymbionts. In most other systems male-killing is observed to occur at around 5–10% in host populations (Hurst & Jiggins, 2000). Exceptions include the Acraea butterflies, where male-killing Wolbachia can generate very highly skewed population sex ratios (Hurst et al., 1999; Jiggins et al., 2000), with many females going unmated as a consequence. In B. tessellatus, it is not known if infection results in lowered mating frequencies or female lifetime fecundity as a consequence of sperm limitation. However, the observed parthenogenesis in this system may reflect selection for female reproduction in the absence of males.
This is only the second documented example of a male-killing Rickettsia bacterium; the other known species is the AB bacterium that causes SRD in the ladybird beetle (Werren et al., 1994), and AB is a close relative of the Btess Rickettsia (Fig. 2). At this time it is not known if the other closely related Rickettsia species that infect the pea aphid and the bruchid beetle, Kytorhinus sharpianus (Fukatsu & Shimada, 1999) (Fig. 2) also cause male-killing. Given their close evolutionary relationships a study of their effects would seem timely.
It appears that SRD in B. tessellatus has been maintained over relatively large geographical and temporal scales. A survey of museum collections of Brachys tessellatus at the Clemson University Arthropod collection indicated skewed sex ratios as far back as the 1930s. In addition, geographical surveys conducted in 1995–99 found evidence of SRD at the population level for all collections (>20) made in South Carolina (Mousseau, unpublished data).
There is a possibility that competition for food in Brachys tessellatus could contribute to the transmission of Rickettsia horizontally, as well as vertically. In high-density populations, more eggs are laid per leaf than can be maintained by that leaf after larvae hatch (Waddell & Mousseau, 1996). We have noted that usually only one larva survives per leaf to adulthood and tends to consume any other embryos laid on the same leaf while developing through its larval instars (Waddell, 1996; Lawson, unpublished data). In this way, the endosymbiont could be transferred horizontally between different families, thus propagating endosymbionts in that population.
The B. tessellatus system is unusual in the degree of variation in sex ratio distortion seen across populations (although see Zakharov et al., 1999; for a discussion of variation in Harmonia axyridis). Populations vary greatly with regards to the degree of SRD (Fig. 1), suggesting either variation among populations in their response to bacterial infection, variation in transmission rates, or both. If cannibalism is the primary means for horizontal transfer one might expect a relationship between population density, the incidence of infection, the degree of male-killing, and the degree of SRD. Tests for such a relationship are presently underway.
One intriguing possible consequence of high frequencies of male-killing bacteria in B. tessellatus could be selection for parthenogenetic development. Twenty-nine percent of females collected from the field produce a low level of offspring parthenogenetically. If males are sufficiently scarce such that females often go uninseminated, this could provide selection for parthenogenetic reproduction. Therefore, the B. tessellatus system could be a system where ‘genetic conflict’ between cytoplasmic sex ratio distorters and nuclear genes leads to selection for changes in the sex determining system (Juchault et al., 1993; Werren & Beukeboom, 1998).
Many of the populations of Brachys tessellatus included in the present study display extremely high population densities (mark–recapture assays indicate peak numbers of adults often exceed 1000 per tree; Lawson, unpublished data), which leads to food limitation and density-dependant survival. Given the likelihood that siblings are often in direct competition with each other under these high density conditions, male-killing may confer a fitness advantage to infected sibling females (i.e. local resource competition). Such a system could in fact generate a positive feedback loop whereby the spread of the male-killer bacterium increases egg lethality, which selects for more laying of multiple eggs on leaves, which in turn increases the fitness advantages of infection. However, the advantages of infection resulting from local resource competition cannot be sustained indefinitely in an obligately sexual diploid organism. At some point a rarity of males must also confer a fitness cost and would select for either alternative reproductive strategies (e.g. parthenogenesis, as appears to be evolving in this system), or the evolution of nuclear suppressors of infection as has been observed for Drosophila (Mercot et al., 1995). The observed variation among populations and families in the Brachys tessellatus system presented here may provide exciting opportunities to further explore the evolutionary dynamics of genetic conflict over sex determination in a natural system.
References
Balas, M. T., Lee, M. H. and Werren, J. H. (1996). Distribution and fitness effects of the son-killer bacterium in Nasonia. Evol Ecol, 10: 593–607.
Chen, D. Q., Campbell, B. C. and Purcell, A. H. (1996). A new rickettsia from a herbivorous insect, the pea aphid Acyrthosiphon pisum (Harris). Curr Microbiol, 33: 123–128, 10.1007/s002849900086.
Dumler, J. S. (1997). Is human granulocytic ehrlichiosis a new Lyme disease? Review and comparison of clinical, laboratory, epidemiological, and some biological features. Clin Infectious Diseases, 25: S43–S47.
Dunn, A. M. and Hatcher, M. J. (1997). Prevalence, transmission and intensity of infection by a microsporidian sex ratio distorter in natural Gammarus duebeni populations. Parasitology, 114: 231–236, 10.1017/s0031182096008475.
Fukatsu, T. and Shimada, M. (1999). Molecular characterization of Rickettsia sp. in a bruchid beetle, Kytorhinus sharpianus (Coleoptera: Bruchidae). Appl Entomol Zool, 34: 391–397.
Gherna, R. L., Werren, J. H., Weisburg, G. W., Cote, R., Woese, C. R. et al (1991). Arsenophonus nasoniae, gen-nov, sp-nov, the causative agent of the son-killer trait in the parasitic wasp Nasonia vitripennis. Int J Syst Bacteriol, 41: 563–565.
Gomi, K., Gotoh, T. and Noda, H. (1997). Wolbachia having no effect on reproductive incompatibility in Tetranychus kanzawai Kishida (Acari: Tetranychidae). Appl Entomol Zool, 32: 485–490.
Hoerauf, A., Volkmann, L., Nissen-Paehle, K., Schmetz, C. et al (2000). Targeting of Wolbachia endobacteria in Litomosoides sigmodontis: comparison of tetracyclines with chloramphenicol, macrolides and ciprofloxacin. Trop Med Int Health, 5: 275–279, 10.1046/j.1365-3156.2000.00544.x.
Hurst, L. D. (1991). The incidences and evolution of cytoplasmic male killers. Proc R Soc B, 244: 91–99.
Hurst, G. D. D. and Jiggins, F. M. (2000). Male-killing bacteria in insects: Mechanisms, incidence, implications. Emerging Infectious Diseases, 6: 329–336.
Hurst, G. D. D., Hurst, L. D. and Majerus, M. E. N. (1997). Cytoplasmic sex ratio distorters. In: O’Neill, S. L. Hoffmann, A. A. and Werren, John H. (eds) Influential Passengers: Inherited Microorganisms and Arthropod Reproduction, p. 152. Oxford University Press, Oxford, 214 pp.
Hurst, G., Jiggins, F. M., Graf von Der Schulenburg, J. H., Bertrand, D. et al (1999). Male killing Wolbachia in two species of insects. Proc R Soc B, 266: 735–740.
Jiggins, F. M., Hurst, G. D. D., Dolman, C. E. and Majerus, M. E. N. (2000). High-prevalence male-killing Wolbachia in the butterfly Acraea encedana. J Evol Biol, 13: 495–501, 10.1046/j.1420-9101.2000.00180.x.
Juchault, P., Rigaud, T. and Mocquard, J. P. (1993). Evolution of sex determination and sex ratio variability in wild populations of Armadillidium vulgare (Latr.) (Crustacea, Isopoda): a case study in conflict resolution. Acta Oecologica, 14: 547–562.
Mercot, H., Atlan, A., Jacques, M. and Montchamporeau, C. (1995). Sex-ratio distortion in Drosophila simulans – cooccurrence of a meiotic drive and a suppressor of drive. J Evol Biol, 8: 282–300.
Moran, N. A., Munson, M. A., Baumann, P. and Ishikawa, H. (1993). A molecular clock in endosymbiont bacteria calibrated using the insect hosts. Proc Natl Acad Sci USA, 253: 167–171.
Myers, S. A. and Sexton, D. J. (1994). Dermatological manifestations of arthropod borne diseases. Infect Dis Clinics North Am, 8: 689–712.
Ochman, H. and Wilson, A. C. (1987). Evolution in bacteria–evidence for a universal substitution rate in cellular genomes. J Mol Evol, 26: 74–86.
Ochman, H., Elwyn, S. and Moran, N. A. (1999). Calibrating bacterial evolution. Proc Natl Acad Sci USA, 96: 12638–12643, 10.1073/pnas.96.22.12638.
Philip, R. N., Casper, E. A., Anacker, R. L., Cory, J. et al (1983). Rickettsia bellii, sp. nova – a tick-borne rickettsia, widely distributed in the United States, that is distinct from the spotted fever and typhus biogroups. Int J Syst Bacteriol, 33: 94–106.
Roux, V. and Raoult, D. (1995). Phylogenetic analysis of the genus Rickettsia by 16s rDNA sequencing. Res Microbiol, 146: 385–396, 10.1016/0923-2508(96)80284-1.
Turcinov, D., Kuzman, I. and Herendic, B. (2000). Failure of azithromycin in treatment of Brill–Zinsser disease. Antimicrobial Agents Chemotherapy, 44: 1737–1738.
Turnbow, R. H. and Franklin, R. T. (1981). Bionomics of Brachys tessellatus in coastal plain scrub oak communities. Ann Entomol Soc Am, 74: 351–358.
Waddell, K. J. (1996) Variables That Influence the Relationship Between Oviposition Preference and Offspring Performance in a Leafmining Beetle. Doctoral Dissertation. University of South Carolina, Columbia, SC.
Waddell, K. J. and Mousseau, T. A. (1996). The oviposition preference hierarchy of a leafmining beetle, Brachys tessellatus (Coleoptera: Buprestidae). Environ Entomol, 25: 63–67.
Weisburg, W. G., Dobson, M. E., Samuel, J. E., Dasch, G. A. et al (1989). Phylogenetic diversity of the Rickettsiae. J Bacteriol, 171: 4202–4206.
Weisburg, W. G., Barns, S. M., Pelletier, D. A. and Lane, D. J. (1991). 16s ribosomal DNA amplification for phylogenetic study. J Bacteriol, 173: 697–703.
Werren, J. H. (1987). Labile sex-ratios in wasps and bees. Bioscience, 37: 498–506.
Werren, J. H. and Beukeboom, L. W. (1998). Sex determination, sex ratios, and genetic conflict. Ann Rev Ecol Syst, 29: 233–261.
Werren, J. H. and O'neill, S. (1997). The evolution of heritable symbionts. In: O’Neill, S. L. Hoffmann, A. A. and Werren, John H. (eds) Influential Passengers: Inherited Microorganisms and Arthropod Reproduction. Oxford University Press, p. 1–41, New York.
Werren, J. H. and Windsor, D. M. (2000). Wolbachia infection frequencies in insects: evidence of a global equilibrium. Proc R Soc B, 267: 1277–1285.
Werren, J. H., Hurst, G. D. D., Zhang, W., Breeuwer, J. A. J. et al (1994). Rickettsial relative associated with male killing in the ladybird beetle (Adalia bipunctata). J Bacteriol, 176: 388–394.
Werren, J. H., Zhang, W. and Guo, L. R. (1995a). Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc R Soc B, 261: 55–71.
Werren, J. H., Windsor, D., Guo, Li Rong (1995b). Distribution of Wolbachia among neotropical arthropods. Proc R Soc B, 262: 197–204.
Williamson, D. L., Sakaguchi, B., Hackett, K. J., Whitcomb, R. F. et al (1999). Spiroplasma poulsonii sp. nov., a new species associated with male-lethality in Drosophila willistoni, a neotropical species of fruit fly. Int J System Bacteriol, 49: 611–618.
Yunker, C. E. (1996). Heartwater in sheep and goats: a review. Onderstepoort J Vet, 63: 159–170.
Zakharov, I. A., Zinkevich, N. S., Shaikevich, E. V., Vysotskaya, L. V. et al (1999). Sex ratio and male killing in Siberian populations of Harmonia axyridis Pall. Russ J Genet, 35: 653–657.
Acknowledgements
We thank the Clemson University Sandhills Research and Education Center, the Sesquicentennial State Park, and the Savannah River Ecology Laboratory for providing field sites for this research. This work could not have been done without a small army of undergraduate and high school summer assistants including Kenn White, Jaime Brown, Tammy McDonald, Angela Smith, Kristin Gissendanner, Amy Desai, Sejal Shah, Jaime Collins, Evan Meadors, Baron Short, Lloyd Raleigh, and Elizabeth Mack. We also thank C. Kennedy and J. Bartos (Werren laboratory) for assistance in molecular characterization of the bacterium, and Dr John Morse of Clemson University for help with the survey of museum specimens. The work of JHW was supported by research grant US-2574-95C from BARD, the United States – Israel Binational Agricultural Research and Development Fund. The work of TAM was supported in part by NSF 9409004, and grants to support undergraduate and high school interns from the SC Governor’s School for Science and Mathematics, the SC AMP program, the USC Howard Hughes program, the SC EPSCoR program, the USC Honors College, Sigma Xi, and the USC School of the Environment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lawson, E., Mousseau, T., Klaper, R. et al. Rickettsia associated with male-killing in a buprestid beetle. Heredity 86, 497–505 (2001). https://doi.org/10.1046/j.1365-2540.2001.00848.x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1046/j.1365-2540.2001.00848.x
Keywords
This article is cited by
-
Why put all your eggs in one basket? Evolutionary perspectives on the origins of monogenic reproduction
Heredity (2023)
-
Turning the tide on sex and the microbiota in aquatic animals
Hydrobiologia (2023)
-
Microbiome analyses of 12 psyllid species of the family Psyllidae identified various bacteria including Fukatsuia and Serratia symbiotica, known as secondary symbionts of aphids
BMC Microbiology (2022)
-
High contiguity de novo genome assembly and DNA modification analyses for the fungus fly, Sciara coprophila, using single-molecule sequencing
BMC Genomics (2021)
-
Genetic and endosymbiotic diversity of Greek populations of Philaenus spumarius, Philaenus signatus and Neophilaenus campestris, vectors of Xylella fastidiosa
Scientific Reports (2021)