Abstract
Purpose
Vasculogenic mimicry patterns, formed by highly invasive melanoma cells, connect to endothelial cell-lined blood vessels and contain fluid in vitroand in vivo. This study was designed to determine if fluid leaks into vasculogenic mimicry patterns without circulation, or if fluid circulates in and clears from these patterns.
Methods
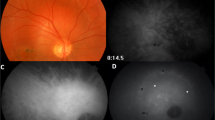
Indocyanine green (ICG) laser scanning confocal angiography (Heidelberg Retinal Angiograph (HRA); Heidelberg Engineering, Heidelberg, Germany) was performed on nine patients with posterior choroidal melanoma in an institutional setting. Blood was drawn before the ICG injection and from the contralateral arm of the ICG injection site and 1 min after the injection. Outcome measures include time to first filling of retinal vessels and vasculogenic mimicry patterns and the time at which no fluorescence could be detected by the HRA instrument. After fluorescence was no longer detected in vessels or patterns, the tubes containing the patient's blood was imaged by the Heidelberg HRA.
Results
Looping vasculogenic mimicry patterns were detected focally in five patients within 30 s after injection and were detectable up to 12 min post-injection. Blood drawn before ICG injection did not autofluoresce but ICG-containing blood pooled in the tube continued to fluoresce at 1-month post-injection.
Conclusions
Vasculogenic mimicry patterns are not part of the endothelial cell-lined vascular system and fluid enters these patterns through leakage. The rapid infusion of ICG into these patterns after injection and the disappearance of fluorescence detectable by the Heidelberg HRA suggest that fluid circulates in these patterns and does not accumulate as a stagnant pool.
Similar content being viewed by others
Introduction
The microcirculation of uveal melanoma is heterogeneous and complex. Lacking lymphatics,1, 2 uveal melanomas contain choroidal vessels that have been incorporated into the tumor, mosaic vessels (composed of vessels lined by tumor cells and endothelial cells), angiogenic vessels, and looping vasculogenic mimicry patterns.2 Highly invasive and genetically deregulated melanoma cells generate patterns that loop around clusters of melanoma cells without participation by endothelial cells or fibroblasts.3, 4 These patterns are rich in laminin,2, 5 fibronectin,6 collagens IV7 and VI,8 and a variety of glycosaminoglycans.9, 10 In human uveal melanoma tissue, vasculogenic mimicry patterns contain only trace amounts of collagen I and are clearly different from fibrovascular septa.6
Non-endothelial cell-lined vasculogenic mimicry patterns have been shown to conduct fluid in vitro3, 4 and in animal models.5, 11 These patterns have also been visualized in patients with posterior choroidal melanomas by laser scanning confocal ophthalmoscopy with indocyanine green (ICG).12, 13
The histological detection of looping vasculogenic mimicry patterns has been associated with an aggressive clinical course in multiple independent studies.14, 15, 16, 17, 18, 19 The presence of these patterns is associated with monosomy 320 (a cytogenetic marker of aggressive uveal melanoma behavior20, 21) and a gene expression signature that is associated with the development of metastatic uveal melanoma.22 The clinical detection of these patterns by confocal ICG angiography has been associated with the eventual growth of small and indeterminate posterior choroidal melanocytic lesions.23
Vasculogenic mimicry patterns, functioning as a ‘fluid-conducting meshwork’5 may also provide an alternative pathway to deliver therapeutic agents to uveal melanomas. Although fluid leaks from the endothelial cell-lined microcirculation into the non-endothelial cell-lined patterned extracellular matrix,4, 11 it is not known if fluid leaks and remains stagnant within vasculogenic mimicry patterns, or if fluid circulates through these patterns.24 This study was designed to test the hypothesis that fluid circulates through vasculogenic mimicry patterns in posterior choroidal melanomas.
Methods
Ten consecutive patients with posterior choroidal melanoma, seen for the first time in the Ophthalmic Oncology Unit of the Hadassah-Hebrew University Medical Center between July 2005 and April 2006, were invited to undergo ICG angiography imaged with a Heidelberg Retinal Angiograph (HRA; Heidelberg Engineering, Heidelberg, Germany). One patient declined to participate because of sensitivity to iodine.
A volume of 3 ml of blood was drawn from the left antecubital vein into a tube without additives immediately before the intravenous injection of ICG, 25 mg/cm3 of sterile aqueous solvent, at that site. For the first 30 s of the study, the angiographer attempted to identify vasculogenic mimicry patterns as defined by three back-to-back loops in each tumor. At 30 s, images were recorded at 10° or 20°, 1–2 frames per second for 3 min, and then at least every 30 s until no fluorescence was detected within the tumor microcirculation. At 1-min post-injection, 3 ml of the patient's blood was drawn from the right antecubital vein into a tube without additives and was stored in the dark at room temperature along with blood drawn before the injection of ICG. After the time point when no fluorescence was detectable with the HRA within the tumor, the patient's blood (pre- and post-injection) was imaged with the HRA at the same settings used for the confocal angiogram. The protocol is summarized in Table 1.
Informed consent was obtained. Digital images were stripped of all identifying (health protected) information and sent to the Department of Pathology at the University of Illinois at Chicago (RF and JL) for identification of vasculogenic mimicry patterns. The thickness of vasculogenic mimicry patterns was calculated from the 20° × 20° angiograms using methods outlined previously by Mueller et al.13 Briefly, at a pixel resolution of 512 × 512 pixels, one pixel equals 11 × 11 μm for a 20° image.
This protocol was approved by the Helsinki Committee of the Hadassah-Hebrew University Medical Center and the Institutional Review Board of the University of Illinois at Chicago and was conducted in accord with HIPAA regulations and the tenets of the Declaration of Helsinki.
Results
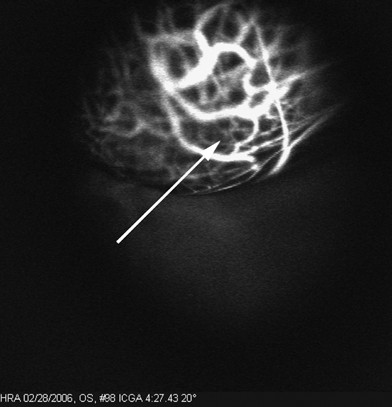
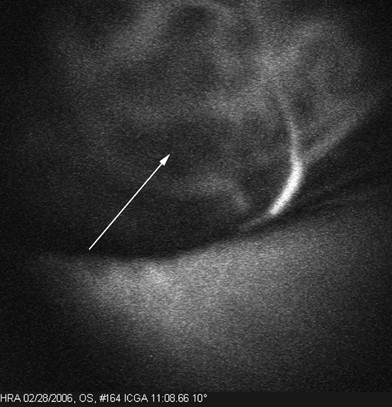
Three back-to-back loops were detected in the tumors of five of nine patients studied. Within 12 min after injection, no fluorescence was detected within either the normal choroidal vessels within the tumor or within vasculogenic mimicry patterns. Figures 1, 2 and 3 illustrate a time-course sequence for one of these patients. No fluorescence was detected in blood drawn from patients before the injection of ICG. However, patient blood, drawn 1 min after injection, was fluorescent at 12 min post-injection, and continued to fluorescence for up to 4 weeks post-angiography. The thickness of the blood column in looping vasculogenic mimicry patterns was measured in this patient at 33 μm, whereas the thickness of normal choroidal vessels in the same frame measured 253 μm. For the five patients whose tumors contained vasculogenic mimicry patterns, the mean thickness of the blood column in the patterns was 37.4 μm (SD 9.8) and the mean thickness of the blood column in the intralesional choroidal vessels was 233.2 μm (SD 47). In this limited data set, the differences between the thicknesses of vasculogenic mimicry loops and normal choroidal vessels approaches significance (P=0.0005 for the paired t-test; P=0.0625 for the sign test).
A 10° × 10° confocal ICG image of the same tumor from Figure 1 taken at 11:08 min after the ICG injection. No fluorescence is visible within the vasculogenic mimicry loops (arrow).
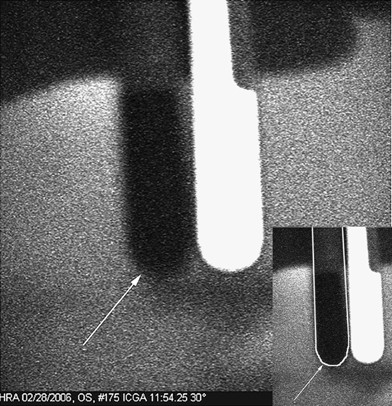
Blood was drawn from the patient 1 min before and after the ICG injection. A 30° × 30° confocal ICG image taken was taken with the HRA at 11:54 min after the ICG injection under the same condition as the angiogram. Blood drawn before the ICG injection (arrow) does not fluoresce. Blood drawn after the injection, positioned to the right of the non-fluorescent tube, continued to fluoresce for 4 weeks after the injection. In the insert, the outline of the tube that contains blood that did not fluoresce is traced in white.
Discussion
Looping vasculogenic mimicry patterns have been shown to connect to endothelial cell-lined blood vessels in histological sections of human primary and metastatic uveal melanomas.4, 25 These patterns are not blood vessels. Three-dimensional reconstructions of vasculogenic mimicry patterns reveal them to be sheets of extracellular matrix proteins – especially rich in laminin – that wrap around branching cylinders of tumor cells.25, 26 The patterns are generated by melanoma cells and are not lined by endothelial cells.3, 4 Because it has been shown repeatedly in animal models that intravenous tracers circulate not only through blood vessels but also through the non-endothelial cell-lined vasculogenic mimicry patterns, 5, 9, 11 it has been proposed that fluid leaks from tumor vessels into the patterns.4, 11 However, it is not known if the fluid that leaks into these patterns stagnates within them or circulates.
Mueller et al13 demonstrated the presence of ICG within looping vasculogenic mimicry patterns in posterior choroidal melanomas by laser scanning ICG confocal angiography and correlated the angiographic pattern detection with the histological detection of looping periodic acid-Schiff (PAS)-positive patterns. Looping vasculogenic mimicry patterns are not normally detected in choroidal nevi. The blood column within these patterns tends to be considerably thinner than that seen in any choroidal vessel. Thus, it is unlikely that the patterns detected by ICG represent normal choroidal vasculature entrapped within uveal melanomas. In a prospective study, angiographic demonstration of these patterns in patients with posterior choroidal melanocytic lesions was associated with the subsequent growth of smaller indeterminate lesions.23
The angiographic detection of ICG within vasculogenic mimicry patterns does not address the issue of whether fluid leaks into the non-endothelial cell-lined extracellular matrix and remains stagnant, or if fluid circulates within the patterns. In this study, we observed that fluorescence within vasculogenic mimicry patterns dissipated within 12 min after intravenous injection of ICG, but blood drawn 1 min after the injection of ICG continued to fluoresce for up to 4 weeks post angiography. These data provide indirect evidence of the circulation of plasma through vasculogenic mimicry patterns in human posterior choroidal melanomas. The presence of an active circulation through vasculogenic mimicry patterns is significant because it has been shown recently that these patterns provide for at least an 11-fold increase in surface area over tumor blood vessels.26 Therefore, vasculogenic mimicry patterns may provide for a more effective delivery route of therapeutic agents than endothelial cell-lined blood vessels.
References
Clarijs R, Schalkwijk L, Ruiter DJ, de Waal RMW . Lack of lymphangiogenesis despite coexpression of VEGF-C and its receptor Flt-4 in uveal melanoma. Invest Ophthal Vis Sci 2001; 42: 1422–1428.
Chen X, Maniotis AJ, Majumdar D, Pe'er J, Folberg R . Uveal melanoma cell staining for CD34 and assessment of tumor vascularity. Invest Ophthalmol Vis Sci 2002; 43: 2533–2539.
Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LMG, Pe'er J et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol 1999; 155: 739–752.
Maniotis AJ, Chen X, Garcia C, DeChristopher PJ, Wu D, Pe'er J et al. Control of melanoma morphogenesis, endothelial survival, and perfusion by extracellular matrix. Lab Invest 2002; 82: 1031–1043.
Clarijs R, Otte-Holler I, Ruiter DJ, de Waal RM . Presence of a fluid-conducting meshwork in xenografted cutaneous and primary human uveal melanoma. Invest Ophthalmol Vis Sci 2002; 43: 912–918.
Lin AY, Maniotis AJ, Valyi-Nagy K, Majumdar D, Setty S, Kadkol S et al. Distinguishing fibrovascular septa from vasculogenic mimicry patterns. Arch Pathol Lab Med 2005; 129: 884–892.
Hao XS, Sun BC, Zhang SW, Zhao XL . Correlation between the expression of collgen IV, VEGF and vasculogenic mimicry. Zhonghua Zhong Liu Za Zhi 2003; 25: 524–526.
Daniels KJ, Boldt HC, Martin JA, Gardner LM, Meyer M, Folberg R . Expression of Type VI collagen in uveal melanoma: role in pattern formation and tumor progression. Lab Invest 1996; 75: 55–66.
Potgens AJG, van Altena MC, Lubsen NH, Ruiter DJ, de Waal RMW . Analysis of the tumor vasculature and metastatic behavior of xenografts of human melanoma cell lines transfected with vascular permeability factor. Am J Pathol 1996; 148: 1203–1217.
Smetsers TFCM, van de Westerlo EMA, ten Dam GB, Clarijs R, Versteeg EM et al. Localization and characterization of melanoma-associated glycosaminoglycans: Differential expression of chondroitin and heparan sulfate epitopes in melanoma. Cancer Res 2003; 63: 2965–2970.
Clarijs R, van Dijk M, Ruiter DJ, de Waal RMW . Functional and morphologic analysis of the fluid-conducting meshwork in xenografted cutaneous and primary uveal melanoma. Invest Ophthalmol Vis Sci 2005; 46: 3013–3020.
Freeman WR, Bartsch DU, Mueller AJ, Banker AS, Weinreb RS . Simultaneous indocyanine green and fluorescein angiography using a confocal scanning laser ophthalmoscope. Arch Ophthalmol 1998; 116: 455–463.
Mueller AJ, Bartsch DU, Folberg R, Mehaffey MG, Boldt HC, Meyer M et al. Imaging the microvasculature of choroidal melanomas with confocal indocyanine green scanning laser ophthalmoscopy. Arch Ophthalmol 1998; 116: 31–39.
Folberg R, Rummelt V, Parys-Van Ginderdeuren R, Hwang T, Woolson RF, Pe'er J et al. The prognostic value of tumor blood vessel morphology in primary uveal melanoma. Ophthalmology 1993; 100: 1389–1398.
Sakamoto T, Sakamoto M, Yoshikawa H, Hata Y, Ishibashi T, Ohnishi Y et al. Histologic findings and prognosis of uveal malignant melanoma in Japanese patients. Am J Ophthalmol 1996; 121: 276–283.
Schaling DF, van der Pol JP, Schlingemann RO, Parys-Van Ginderdeuren R, Jager MJ . In: Schaling DF. (ed) Vascular density and vascular patterns in the prognosis of choroidal melanoma. In: Radionucleotides and Radiolabelled Antibodies in Choroidal Melanoma (Diagnosis and Therapy). Rijksuniversiteit te Leiden: Leiden, 1996.
McLean IW, Keefe KS, Burnier MN . Uveal melanoma: comparison of the prognostic value of fibrovascular loops, mean of the ten largest nucleoli, cell type and tumor size. Ophthalmology 1997; 104: 777–780.
Seregard S, Spangberg B, Juul C, Oskarsson M . Prognostic accuracy of the mean of the largest nucleoli, vascular patterns, and PC-10 in posterior uveal melanoma. Ophthalmology 1998; 105: 485–491.
Makitie T, Summanen P, Tarkkanen A, Kivela T . Microvascular density in predicting survival of patients with choroidal and ciliary body melanoma. Invest Ophthalmol Vis Sci 1999; 40: 2471–2480.
Scholes AGM, Damato BE, Nunn J, Hiscott P, Grierson I, Field JK . Monosomy 3 in uveal melanoma: Correlation with clinical and histologic predictors of survival. Invest Ophthalmol Vis Sci 2003; 44: 1008–1011.
Prescher G, Bornfeld N, Hirche H, Horsthemke B, Jockel KH, Becher R . Prognostic impliations of monosomy 3 in uveal melanoma. Lancet 1996; 347: 1222–1225.
Onken MD, Lin AY, Worley LA, Folberg R, Harbour JW . Association between microarray gene expression signature and extravascular matrix patterns in primary uveal melanomas. Am J Ophthalmol 2005; 140: 748–749.
Mueller AJ, Schaller U, Freeman W, Folberg R, Kampik A . Complex microcirculation patterns detected by confocal indocyanine green angiography predict time to growth of small choroidal melanocytic tumors. MuSIC-Report II. Ophthalmology 2002; 109: 2207–2214.
McDonald DM, Munn L, Jain RK . Vasculogenic mimicry: how convincing, how novel, and how significant? Am J Pathol 2000; 156: 383–388.
Chen X, Ai Z, Rasmussen M, Bajcsy P, Auvil L, Welge M et al. Three-dimensional reconstruction of extravascular matrix patterns and blood vessels in human uveal melanoma tissue: techniques and preliminary findings. Invest Ophthalmol Vis Sci 2003; 44: 2834–2840.
Lin AY, Ai Z, Lee SC, Bajcsy P, Pe'er J, Leach L et al. Comparing vasculogenic mimicry with endothelial cell lined vessels: techniques for 3D reconstruction and quantitative analysis of tissue components from archival paraffin blocks. Appl Immunohistochem Mol Morphol 2007; 15: 113–119.
Acknowledgements
Supported by National Eye Institute Grant EY10457 (RF) and EY016323 (DUB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented in part at the 2006 ARVO meeting (E-Abstract 1494). Fort Lauderdale, Florida, USA
Rights and permissions
About this article
Cite this article
Frenkel, S., Barzel, I., Levy, J. et al. Demonstrating circulation in vasculogenic mimicry patterns of uveal melanoma by confocal indocyanine green angiography. Eye 22, 948–952 (2008). https://doi.org/10.1038/sj.eye.6702783
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702783
Keywords
This article is cited by
-
Glioblastoma vasculogenic mimicry: signaling pathways progression and potential anti-angiogenesis targets
Biomarker Research (2015)
-
A pilot histomorphology and hemodynamic of vasculogenic mimicry in gallbladder carcinomas in vivo and in vitro
Journal of Experimental & Clinical Cancer Research (2011)
-
Tumor angiogenesis: molecular pathways and therapeutic targets
Nature Medicine (2011)
-
Tumour vascularization: sprouting angiogenesis and beyond
Cancer and Metastasis Reviews (2007)