Abstract
Purpose
To determine the most cost-effective case-finding strategy for the ophthalmologist to detect and treat ocular hypertension (OH) and primary open-angle glaucoma (POAG) at an early stage to prevent blindness.
Design
A Markov cost-effectiveness simulation model.
Methods
Three case-finding strategies are analysed and compared. The simulated cohort consists of all initial patients of at least 40 years old visiting an ophthalmic practice. All patients undergo ophthalmoscopy, but tonometry is routinely performed to: (1) all initial patients, (2) high-risk patients only, or (3) no one. The population characteristics are based on data of 1000 initial patients. Transition probabilities are taken from the literature. The (direct) costs of diagnosis and treatment represent those for the Netherlands. The time-horizon of the model is 20 years. An annual discount rate of 4% is used.
Main Outcome Measures
Costs, proportion of patients becoming blind, years of blindness.
Results
The costliest strategy (1) leads to least blindness. The incremental cost-effectiveness ratio, which shows extra costs per year of vision saved in comparison to the cheapest strategy (3), is lower for strategy (1) than for strategy (2). It amounts to €1707, not including extra costs due to blindness (eg associated with the use of disability facilities). When such costs exceed €1707 per patient per year, which is most likely, then strategy (1) becomes cost saving.
Conclusion
It is most cost-effective to routinely perform tonometry to all initial ophthalmic patients to prevent blindness due to glaucoma.
Similar content being viewed by others
Introduction
Primary open-angle glaucoma (POAG) is an important public health problem, being the second leading cause of bilateral blindness worldwide.1 It is characterised by progressive optic nerve atrophy leading to visual field damage and possibly blindness. The natural history of POAG is though variable; some people develop significant visual problems, while others do not.2 An elevated intraocular pressure (IOP) defines ocular hypertension (OH) and is a major risk factor for POAG. Through IOP lowering treatment the risk of glaucoma and its progression can be reduced.3, 4
In the past it has often been suggested that a screening programme should be set up, but because of high costs and unknown effectiveness it has never been systematically introduced.5 In the last two decades new technologies have been introduced that permit earlier detection of structural and functional damage due to glaucoma. New treatments, such as hypotensive lipids and selective laser trabeculoplasty, allow for safer and more effective IOP reduction. Several well-conducted clinical trials showed the effectiveness of treatment.3, 4 These new developments, and a possibly increasing prevalence of POAG due to ageing, bring along the necessity to re-evaluate screening.6 However, the feasibility and efficacy of a screening programme still can be questioned. Only recently the US Preventive Services Task Force concluded that there is still insufficient evidence to recommend for or against a screening programme for glaucoma.7, 8
An alternative for screening to discover and treat OH and POAG at an early stage is case-finding among patients visiting an ophthalmologist. The advantages compared to screening in the general population are the use of an existing infrastructure, the high probability for discovery by means of a complete ophthalmic examination, and the direct initiation of treatment without loss to follow-up. Moreover, the prevalence of OH, POAG, and risk factors for glaucoma, is higher among patients visiting an ophthalmologist than in the general population.
In many countries ophthalmologists routinely apply strategies to detect OH and POAG. However, the effect of these strategies to prevent glaucoma blindness is unknown. The cost-effectiveness is unknown as well. This study aims to investigate the effects, costs, and cost-effectiveness of different case-finding strategies that can be applied by an ophthalmologist in daily practice to detect and treat OH and POAG at an early stage to prevent glaucoma blindness.
Methods
Three case-finding strategies, which differ with respect to the group of patients routinely receiving tonometry, are analysed and compared using a Markov cost-effectiveness simulation model. In the model all initial ophthalmic patients undergo ophthalmoscopy to detect a glaucomatous optic nerve, but the three strategies differ by whether tonometry is routinely performed to: (1) all initial patients, (2) high-risk patients only, or (3) no one. In case of positive results of testing with ophthalmoscopy and/or tonometry, perimetry is performed to complete the assessment of the correct diagnosis. These different diagnostic strategies lead to different proportions of patients being diagnosed and treated for OH or POAG. The long-term consequences of these three strategies are then evaluated.
The population characteristics are based on data gathered from the charts of 1000 initial patients visiting an ophthalmic practice. The transition probabilities are taken from the literature. The (direct) costs of diagnosis and treatment represent those for the Netherlands. The time-horizon of the model is 20 years and an annual discount rate of 4% is used. The analysis is performed from a societal perspective.
The model
It is modelled that a cohort of initial patients visiting an ophthalmologist, having a certain prevalence of OH and POAG, undergoes testing to detect these disorders. The use of the diagnostic tests is strategy dependent, as described above, and so per strategy different proportions of patients are specified for observation, treatment, or discharge. Subsequently a 20-year follow-up is modelled, while the progression of patients returning for regular visits, but also that of the discharged patients is being tracked. Per strategy blindness due to glaucoma is determined and the corresponding costs are computed.
The cohort simulated in the model corresponds to initial patients of at least 40 years old visiting an ophthalmic practice. In the Netherlands the general practitioner refers the majority of such patients. The age and sex distribution, and the prevalence of OH and POAG are jointly taken into account. In the model the high-risk group is defined to consist of patients reporting a positive family history of glaucoma. Such patients have an increased risk of having glaucoma.9, 10
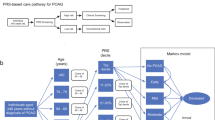
The model consists of two parts. Part one models the diagnostic process, part two models the disease progression over time. In part one, the known sensitivity and specificity of ophthalmoscopy and tonometry are used to calculate proportions of patients receiving the diagnosis OH or POAG for each strategy. This leads to a strategy dependent distribution of the patient cohort over the set of health states. These health states are defined by the true condition of a patient, by the obtained diagnosis and by the assigned therapy (see Figure 1). The therapy assignment corresponds to the clinical practice, where IOP level, the presence of risk factors, and severity of the disease play a role. Patients diagnosed as OH are either put under observation or given medical treatment with monotherapy. In the strategy ‘tonometry all’ 50% of the OH patients starts medication directly after the diagnosis is made. The rest is kept under observation and receives treatment only if conversion to POAG occurs. In the strategy ‘tonometry high-risk group’ all patients diagnosed as OH are at risk and therefore require medication. POAG patients start therapy with monotherapy in all strategies; a small proportion undergoes laser or surgery directly. Missed OH and POAG patients are discharged.
Part two of the simulation model: transitions among the health states. The health state ‘dead’ (not shown) can be entered from all other states. After the diagnosis was made, in part one of the model, all health states except ‘blind’ and ‘dead’ can be entered. The shown scheme is a simplified reproduction of the model. In the model, a distinction is namely made between undiscovered patients completely discharged and patients still visiting the ophthalmologist regularly for some other ophthalmic condition. A distinction is also made in the form of medication, namely monotherapy, or combination therapy. Another health state, not shown, is ‘false positives on monotherapy’.
In part two of the model, transitions between health states can take place. Here, the development of OH in normotensives, the development of POAG from OH, and the progression of POAG to blindness is modelled. Also, undiagnosed patients are tracked and can be rediscovered. A distinction is made between patients completely discharged and patients still visiting the ophthalmologist regularly for some other ophthalmic condition. The latter group of patients has a higher chance of OH/POAG detection. For patients under therapy every 6 months an outpatient visit is modelled to take place and the proportion of patients showing progression is modelled to undergo a therapy switch. The therapy sequence is based on recommendations of the American Academy of Ophthalmology; its chronological order is monotherapy, combination therapy, laser, and surgery.11 The end stage of the disease is blindness. Patients under treatment develop blindness only after surgery, when there are no further treatment possibilities. Discharged patients can also eventually develop blindness without treatment. Patients who become blind continue to use combination therapy and to visit an ophthalmologist once in 2 years. The false positives, patients unjustly positively diagnosed, continue to use monotherapy medication.
The model is built and analysed using the software package ‘Decision Analysis by TreeAge’ (Data), version 3.5. It has the structure of a Markov model and it has a time-horizon of 20 years, consisting of 40 cycles of 6 months each. This choice of cycle length reflects the common time between the consecutive outpatient visits of the glaucoma patients. The diagnostic process is implemented as the first cycle only, the disease progression over time involves the other cycles. At the end of each cycle transitions between the health states take place. These transitions include age- and sex-dependent survival probabilities, probabilities concerning the discovery of undiagnosed patients, and the probabilities related to the development or progression of OH and POAG. These probabilities are independent of the three strategies being evaluated.
Cost assignment
The direct medical costs are assigned as once-only costs and state-dependent costs. The once-only costs are the costs of the diagnostic process and the costs for laser treatment and surgery. The state-dependent costs are the costs of outpatient visits and medication. These costs are assigned to the health states and reflect the treatment requirements related to 6 months of treatment of a particular state. The costs for diagnosis apply to all patients. The costs for treatment apply to diagnosed patients only.
Outcomes
The outcomes of the analysis concern: (1) costs, (2) proportion of patients becoming blind, (3) years of blindness.
The data
The data and their sources are presented in Tables 1, 2 and 3. The age distribution and the OH/POAG prevalence for the population have been determined from the charts of 1000 initial patients, consecutively visiting a general ophthalmic practice in Maastricht (Medisch Centrum Maastricht Annadal—MCMA), starting from January 1999.12 The global characteristics of the data are consistent with the indications from the literature.13, 14, 15 The values for sensitivity and specificity of the diagnostic tests were taken from the literature. The starting therapy assignment for discovered patients is consistent with the opinion of the glaucoma specialists, the recommendations of the American Academy of Ophthalmology and the European Glaucoma Society.11, 16 In part two of the model, the 6-month transition probabilities are calculated from the yearly transition probabilities, which are obtained as follows. The age- and sex-dependent survival probabilities are taken from data provided by the Dutch Central Bureau for Statistics (CBS).17 The modelled disease progression is based on the literature, mainly on the long-term follow-up studies and on therapy trials.3, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 The unit prices related to the outpatient visits, laser trabeculoplasty and surgery are as determined at the University Hospital Maastricht. This was performed according to the micro costing method.38 The prices of medical drugs represent the prices in the Netherlands (incl. VAT). The prices of monotherapy and combination therapy are based on the average use of the individual drugs in the Netherlands, combining up to three drugs in the combination therapy. The frequency of the health care use is modelled in accordance with the specialist's opinion and the recommendations of the American Academy of Ophthalmology. The costs due to blindness in connection with the usage of disability facilities in the Netherlands could not be retrieved.
Results
Table 4 gives the expected costs and outcomes per initial ophthalmic patient due to OH and POAG within a period of 20 years. Not surprisingly, both strategies ‘tonometry all’ and ‘tonometry high risk group’ cost more than ‘tonometry no one’ but also avert more blindness. The strategy ‘tonometry all’ is the most expensive one, with least blindness. For this strategy the incremental costs to prevent blindness in comparison with ‘tonometry no one’ are € 7778 to prevent one person from becoming blind and € 1707 per year of vision saved. For strategy ‘tonometry high risk group’ the incremental costs to prevent blindness are higher. These outcomes do not include extra costs due to blindness, for example, in connection with the usage of disability facilities. Since such costs are likely to exceed € 1707 per year, inclusion of such costs makes the strategy ‘tonometry all’ cheapest and most effective.
The sensitivity analysis shows that in strategy ‘tonometry all’ the occurrence of blindness is always lowest and in strategy ‘tonometry no one’ always highest. The incremental costs to prevent blindness are always lower for the strategy ‘tonometry all’ than ‘tonometry high-risk group’. These results show the robustness of the conclusions.
In a one-way sensitivity analysis the lower and upper bound values of all parameters for which ranges are given were used. Alteration of glaucoma incidence among undiscovered OH patients has the largest impact on the results of the cost-effectiveness analysis. The incremental costs per year of vision saved for strategy ‘tonometry all’ are €4670, when glaucoma incidence among discharged OH patients is lowest and €564 when it is highest. Alteration of blindness incidence among untreated glaucoma patients gives incremental costs per year of vision saved €3900, when it is lowest, and €1240, when it is highest. A two-way sensitivity analysis, which uses the lower values of both above-mentioned parameters, gives the incremental costs €12 250 per year of vision saved. Alteration of the health care costs, with all unit prices raised by 10 and 30%, has no substantial effect on the outcomes of the cost-effectiveness analysis (data not shown). Neither does an alteration of the cohort characteristics and of the sensitivity and specificity of the diagnostic tests (data not shown).
Discussion
This study evaluates the long-term consequences of three case-finding strategies, which differ with respect to the group of patients receiving tonometry. For this purpose a simulation model was used. Although a randomised clinical trial would be of interest, it is almost impossible to conduct. Beside the ethical objections such trial would inevitably give large expenses because of the low prevalence of glaucoma and its slow progression to blindness. An advantage of modelling is the possibility to simulate and compare several strategies. Another advantage is the possibility to integrate current research results and examine the influence of alteration of different parameters.
The figures incorporated in the model are based on information from different sources. This could give uncertainty about the exact value of the figures. A sensitivity analysis was performed to assess the impact of variation of the uncertain quantities in the model on the outcomes of the cost-effectiveness analysis. The conclusions were robust to these uncertainties.
To validate the simulation model strategy ‘tonometry all’, which represents more or less the current practice, was studied in more detail. The model shows that on average over a period of 20 years, each year 5.2% of glaucoma patients undergoes laser therapy and 2.7% surgery. A questionnaire, distributed among glaucoma patients in randomly chosen practices in the Netherlands, showed comparable results.39 Per year 4.8% of patients had laser therapy and 2% surgery. The model was further validated by comparing the incidence of glaucoma blindness to those reported in the literature. Two longitudinal studies were conducted in patients from a clinic.40, 41 The incidence rate of blindness in these studies was 1.05 and 0.95% per year, respectively. The model shows an incidence rate of blindness 0.90% per year.
The costs seem to be comparable to those reported in the literature. One study assessed the annual resource use and costs of patients in the Netherlands.42 The mean costs per glaucoma patient were €469 (converted and using the retail price index). In the simulation model this is €454. Another study calculated the direct treatment costs for newly diagnosed OH or POAG patients in Germany.43 The mean costs per OH/POAG patient for 1 year of treatment were about €324 (converted and using the retail price index). The same authors calculated the costs for France and the UK.44 This was €387 and €560, respectively. Other published glaucoma cost studies show comparable estimates for yearly costs in Europe and Canada.45, 46, 47 In the US the published yearly costs ranged from €486 for early stage of the disease to €1960 for patients with end-stage disease.48 Differences in unit costs, in medication prices, in the assignment of laser therapy and surgery, can explain some variations in the costs. However, raising the costs in our study did not affect its conclusions.
The outcomes show that strategy ‘tonometry all’ is the most preferable in relation to blindness prevention. This strategy is most costly, but prevents most blindness. The incremental costs to prevent blindness when compared to ‘tonometry no one’ are small. For strategy ‘tonometry high risk group’ the incremental costs are higher. Even if the financing would be limited, it would be more preferable to perform tonometry in all patients, and not in high-risk group only, because strategy ‘tonometry all’ dominates strategy ‘tonometry high risk group’ in the extended sense.38 The costs due to blindness from glaucoma could not be retrieved. If such yearly costs exceed €1707, then performing tonometry in all patients becomes cost saving. This is likely the case because blindness is accompanied by the utilisation of disability facilities. In the Netherlands a common help in the household for disabled people (12 h a week) has a yearly cost of €5300. The costs of blindness may vary per country depending on the care and support given. A study conducted in the UK, which took the societal perspective, estimated the costs of blindness for people with age-related macular degeneration.49 The costs of blindness in this study were €10 134 (€2159–€26 847) in the first year and €9883 (€2080–€26 376) in the second and subsequent years (converted and using the retail price index). The same paper gives an overview of the estimates of the cost of blindness for UK, Scotland, Australia, USA, and Switzerland. The yearly costs due to blindness in these countries probably exceed €1707.
At first glance it is not obvious why strategy ‘tonometry all’ is the most cost-effective because in general, approaching a high-risk group would give a higher yield of case finding. However, in strategy ‘tonometry all’ not every detected OH patient is treated, as current guidelines advice only treatment in case of a presence of a risk factor. Those who are not treated at once are followed for the occurrence of glaucoma. Those who develop glaucoma are treated at an early stage; thereby blindness can still be prevented, and the costly treatment of those OH patients who never develop POAG can be avoided. When the proportion of high-risk patients receiving treatment was made similar to that in strategy ‘tonometry all’, the results became more alike, confirming that monitoring of OH patients without direct treatment contributes to a favourable cost-effectiveness ratio of strategy ‘tonometry all’.
The impact of not using the ophthalmoscope to detect POAG has not been addressed. In daily practice ophthalmoscopy cannot be left out of the first ophthalmic examination. Its use is needed for the diagnosis of many diseases. Tonometry could be left out more easily since there is often no indication for it, except for the early detection of OH or POAG. The use of perimetry, costly and time consuming, is unlikely an option for case-finding. In the model patients undergo perimetry on indication, to confirm the diagnosis.
A positive family history of glaucoma was appointed as an indication for being at risk of having glaucoma. It is an established risk factor and it is easy to assess in daily practice.11 It has been estimated that about 20% of the OH and POAG patients and 5% of others in the ophthalmic patients population report a positive family history of glaucoma.10, 50 The simulations show that even if these proportions are much higher strategy ‘tonometry all’ remains the most cost-effective (data not shown).
Note that normal tension glaucoma (NTG) is not included in this study. The role of IOP in its pathogenesis and the response to therapeutic lowering of IOP is different in comparison with high-tension glaucoma. Moreover, this disorder cannot be primarily detected by tonometry. As a consequence, inclusion of NTG in the study has no impact on the outcomes of the cost-effectiveness analysis, because the incremental costs and the incremental effects do not change. When NTG is included, the proportion of patients prevented from blindness due to glaucoma is slightly lower than calculated.
The indirect costs have not been used in the presented analysis, because glaucoma effects mainly elderly and the consequences in terms of potential loss of production are limited.51 No cost-utility analysis has been performed. An estimate for the utility for blindness is 0.6.52 The estimated incremental costs per quality-adjusted life-year (QALY) for strategy ‘tonometry all’ are then approximately €2850. According to the guidelines in the Netherlands a 4% discount rate is used. As of the current international recommendation of 3%, an extra analysis was performed.38, 53 If using a 3% discount rate, the incremental costs per year of vision saved for ‘tonometry all’ are €1550.
Conclusion
The strategy ‘tonometry all’ is the most preferable strategy for blindness prevention, with €1707 extra costs per year of vision saved when compared to strategy ‘tonometry no one’. When costs associated with the use of disability facilities due to blindness exceed €1707 per patient per year, which is most likely, then strategy ‘tonometry all’ becomes cost saving. We conclude that it is most cost-effective to routinely perform tonometry to all initial ophthalmic patients to prevent blindness due to glaucoma.
References
Quigley HA . Number of people with glaucoma worldwide. Br J Ophthalmol 1996; 80: 389–393.
Harris R . Screening for glaucoma. BMJ 2005; 331: 376–377.
Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002; 120: 701–713.
Heijl A, Leske MC, Bengtsson B, Hyman L, Hussein M . Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 2002; 120: 1268–1279.
Gottlieb LK, Schwartz B, Pauker SG . Glaucoma screening. A cost-effectiveness analysis. Survey Ophthalmol 1983; 28: 206–226.
Harasymowycz P, Kamdeu Fansi A, Papamatheakis D . Screening for primary open-angle glaucoma in the developed world: are we there yet? Canad J Ophthalmol 2005; 40: 477–486.
Fleming C, Whitlock EP, Beil T, Smit B, Harris RP . Screening for primary open-angle glaucoma in the primary care setting: an update for the US preventive services task force. Annal Family Med 2005; 3: 167–170.
US preventive services task forces. Screening for glaucoma: recommendation statement. Annals Family Med 2005; 3: 171–172.
Wolfs RC, Klaver CC, Ramrattan RS, van Duijn CM, Hofman A, de Jong PT . Genetic risk of primary open-angle glaucoma. Population-based familial aggregation study. Arch Ophthalmol 1998; 116: 1640–1645.
Tielsch JM, Katz J, Sommer A, Quigley HA, Javitt JC . Family history and risk of primary open angle glaucoma. The Baltimore Eye Survey. Arch Ophthalmol 1994; 112: 69–73.
American Academy of Ophthalmology. Preferred Practice Patterns: Primary Open-Angle Glaucoma. American Academy of Ophthalmology: San Francisco, CA, 2000.
Van der Horst FG, Webers CAB, Bours SJM . Transmuraal Model Oogzorg (Transmural Model Eyecare). Datawyse: Maastricht, 2003.
Koch H . Practice Patterns of the Office-Based Ophthalmologist, National Ambulatory Medical Care Survey, 1985. Adv Data 1989; 162: 1–12.
Schappert SM . Office visits for glaucoma: United States, 1991–92. Advance data 1995; 262: 1–14.
Ellwein LB, Urato CJ . Use of eye care and associated charges among the Medicare population: 1991–1998. Arch Ophthalmol 2002; 120: 804–811.
European Glaucoma Society. Terminology and Guidelines for Glaucoma. Editrice Dogma® S.r.I.: Savona–Italy, 2003.
Centraal Bureau voor de Statistiek (CBS). Statline. Available at:http://statline.cbs.nl. Accessed 8 September 2003.
Jensen JE . Glaucoma screening: a 16-year follow-up of ocular normotensives. Acta Ophthalmol 1984; 62: 203–209.
Perkins SE . Bedford Glaucoma Survey II. Rescreening of the normal population. Br J Ophthalmol 1973; 57: 186–192.
Bengtsson B . Manifest glaucoma in the aged I: occurrence nine years after a population survey. Acta Ophthalmol 1981; 59: 321–331.
Sorensen PN, Nielsen NV, Norskov K . Ocular hypertension. A 15-year follow-up. Acta Ophthalmol 1978; 56: 363–372.
Lundberg L, Wettrell K, Linner E . Ocular hypertension. A prospective twenty-year follow-up study. Acta Ophthalmol 1987; 65: 705–708.
Epstein DL, Krug Jr JH, Hertzmark E, Remis LL, Edelstein DJ . A long-term clinical trial of timolol therapy versus no treatment in the management of glaucoma suspects. Ophthalmology 1989; 96: 1460–1467.
Kamal D, Garway-Heath D, Ruben S, O'Sullivan F, Bunce C, Viswanathan A et al. Results of the betaxolol versus placebo treatment trial in ocular hypertension. Graefe's archive for clinical and experimental ophthalmology 2003; 241: 196–203.
Uusitalo RJ, Palkama A . Long-term evaluation of timolol. Acta Ophthalmol 1989; 67: 573–581.
Airaksinen PJ, Valle O, Takki KK, Klemetti A . Timolol treatment of chronic open-angle glaucoma and ocular hypertension. A 2.5-year multicenter study. Graefe's Arch Clin Exp Ophthalmol 1982; 219: 68–71.
Maclure GM . Chronic open angle glaucoma treated with Timolol. A four year study. Trans Ophthalmol Soc UK 1983; 103: 78–83.
Heijl A, Bengtsson B . Long-term effects of timolol therapy in ocular hypertension: a double-masked, randomised trial. Graefe's Arch Clin Exp Ophthalmol 2000; 238: 877–883.
Shingleton BJ, Richter CU, Bellows AR, Hutchinson BT, Glynn RJ . Long-term efficacy of argon laser trabeculoplasty. Ophthalmology 1987; 94: 1513–1518.
Ticho U, Nesher R . Laser trabeculoplasty in glaucoma. Ten-year evaluation. Arch Ophthalmol 1989; 107: 844–846.
Vogel M, Ecker S, Cieplik G, Bernhardt G . Laser trabeculoplasty in glaucoma chronicum simplex. Results of a prospective, clinical study [in German]. Klinische Monatsblatter fur Augenheilkunde 1988; 193: 8–15.
Ustundag C, Diestelhorst M . Efficacy of argon laser trabeculoplasty: 3-year preliminary results of a prospective placebo-controlled study. Graefe's Arch Clin Exp Ophthalmol 1997; 235: 354–358.
Spiegel D, Wegscheider E, Lund OE . Argon laser trabeculoplasty: long-term follow-up of at least 5 years. German J Ophthalmol 1992; 1: 156–158.
Parc CE, Johnson DH, Oliver JE, Hattenhauer MG, Hodge DO . The long-term outcome of glaucoma filtration surgery. Am J Ophthalmol 2001; 132: 27–35.
Molteno AC, Bosma NJ, Kittelson JM . Otago glaucoma surgery outcome study: long-term results of trabeculectomy—1976 to 1995. Ophthalmology 1999; 106: 1742–1750.
Lamping KA, Bellows AR, Hutchinson BT, Afran SI . Long-term evaluation of initial filtration surgery. Ophthalmology 1986; 93: 91–101.
Popovic V, Sjostrand J . Long-term outcome following trabeculectomy: II Visual field survival. Acta Ophthalmol 1991; 69: 305–309.
Gold MR, Siegel JE, Russel LB et al. Cost-effectiveness in Health and Medicine. First press release. Oxford University Press: New York, 1996.
Voorlichting aan glaucoompatienten (Education for glaucoma patients). Rapport, 2002; nr 143. College voor zorgverzekeringen: Amstelveen, the Netherlands, 2002.
Chen PP . Blindness in patients with treated open-angle glaucoma. Ophthalmology 2003; 110: 726–733.
Kwon YH, Kim CS, Zimmerman MB, Alward WL, Hayreh SS . Rate of visual field loss and long-term visual outcome in primary open-angle glaucoma. Am J Ophthalmol 2001; 132: 47–56.
Oostenbrink JB, Rutten van Molken MP, Sluyter Opdenoordt TS . Resource use and costs of patients with glaucoma or ocular hypertension: a one-year study based on retrospective chart review in the Netherlands. J Glaucoma 2001; 10: 184–191.
Kobelt G, Jonsson L, Gerdtham U, Krieglstein GK . Direct costs of glaucoma management following initiation of medical therapy. A simulation model based on an observational study of glaucoma treatment in Germany. Graefe's Arch Clin Exp Ophthalmol 1998; 236: 811–821.
Kobelt G, Jonsson L . Modeling cost of treatment with new topical treatments for glaucoma. Results from France and the United Kingdom. Int J Technol Assess Health Care 1999; 15: 207–219.
Traverso CE, Walt JG, Kelly SP et al. Direct costs of glaucoma and severity of the disease: a multinational long term study of resource utilisation in Europe. Br J Ophthalmol 2005; 89: 1245–1249.
Lindblom B, Nordmann JP, Sellem E et al. A multicentre, retrospective study of resource utilization and costs associated with glaucoma management in France and Sweden. Acta ophthalmol Scand 2006; 84: 74–83.
Iskedjian M, Walker J, Vicente C, Trope GE, Buys Y, Einarson TR et al. Cost of glaucoma in Canada: analyses based on visual field and physician's assessment. J Glaucoma 2003; 12: 456–462.
Lee PP, Walt JG, Doyle JJ, Kotak SV, Evans SJ, Budenz DL et al. A multicenter, retrospective pilot study of resource use and costs associated with severity of disease in glaucoma. Arch Ophthalmol 2006; 124: 12–19.
Meads C, Hyde C . What is the cost of blindness? Br J Ophthalmol 2003; 87 (10): 1201–1204.
Weih LM, Nanjan M, McCarty CA, Taylor HR . Prevalence and predictors of open-angle glaucoma: results from the visual impairment project. Ophthalmology 2001; 108: 1966–1972.
Kobelt G . Health economics, economic evaluation, and glaucoma. J Glaucoma 2002; 11: 531–539.
Brown MM, Brown GC, Sharma S, Busbee B . Quality of life associated with visual loss: a time tradeoff utility analysis comparison with medical health states. Ophthalmology 2003; 110: 1076–1081.
Hunink MGM, Glasziou PP, Siegel JE, Weeks JC, Pliskin JS, Elstein AS et al. Decision Making in Health and Medicine: Integrating Evidence and Values. Cambridge University Press: Cambridge, 2001.
Dielemans I, Vingerling JR, Hofman A, Grobbee DE, de Jong PT . Reliability of intraocular pressure measurement with the Goldmann applanation tonometer in epidemiological studies. Graefe's Arch Clin Exp Ophthalmol 1994; 232: 141–144.
Grolman B, Myers KJ, Lalle P . How reliable is the Goldmann tonometer as a standard? J Am Optometr Assoc 1990; 61: 857–862.
Whitacre MM, Stein R . Sources of error with use of Goldmann-type tonometers. Survey Ophthalmol 1993; 38: 1–30.
Tielsch JM, Katz J, Singh K et al. A population-based evaluation of glaucoma screening: the Baltimore Eye Survey. Am J Epidemiol 1991; 134: 1102–1110.
Tuck MW, Crick RP . The cost-effectiveness of various modes of screening for primary open angle glaucoma. Ophthalmic Epidemiology 1997; 4: 3–17.
Quigley HA . Open-angle glaucoma. N Engl J Med 1993; 328: 1097–1106.
Harper R, Reeves B . The sensitivity and specificity of direct ophthalmoscopic optic disc assessment in screening for glaucoma: a multivariate analysis. Graefe's Arch Clin Exp Ophthalmol 2000; 238: 949–955.
Ivers RQ, Macaskill P, Cumming RG, Mitchell P . Sensitivity and specificity of tests to detect eye disease in an older population. Ophthalmology 2001; 108: 968–975.
Mass IAM, Gijsen R, Lobbezoo IE, Poos MJJC . De gezondheidstoestand van de bevolking in Nederland: een actualisering, RIVM, Bilthoven (The health condition of the dutch population). Elsevier/De Tijdstroom: Maarssen, the Netherlands, 1997.
Quigley HA . Proportion of those with open-angle glaucoma who become blind. Ophthalmology 1999; 106: 2039–2041.
Jay JL, Murdoch JR . The rate of visual field loss in untreated primary open angle glaucoma. Br J Ophthalmol 1993; 77: 176–178.
Commissie Farmaceutische hulp (CFH) van het College voor zorgverzekeringen (Health Care Insurance Board). Farmacotherapeutisch Kompas (Pharmacotherapeutic Compass) 2004; ISBN 90-70918-35-8.
Acknowledgements
Financial support has been provided by the Dutch Health Care Insurance Council, Diemen, The Netherlands. No author, nor the financer, has any commercial (proprietary or financial) interest in any drugs, devices or techniques discussed in this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper has not been published before. Preliminary results were presented in part as a poster at: ISPOR 10th Annual International Meeting, May 2005; Washington, DC, USA.
Rights and permissions
About this article
Cite this article
Peeters, A., Schouten, J., Webers, C. et al. Cost-effectiveness of early detection and treatment of ocular hypertension and primary open-angle glaucoma by the ophthalmologist. Eye 22, 354–362 (2008). https://doi.org/10.1038/sj.eye.6702637
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702637
Keywords
This article is cited by
-
Contributing ocular comorbidity to end-of-life visual acuity in medically treated glaucoma patients, ocular hypertension and glaucoma suspect patients
Eye (2021)
-
Systematic Review of Economic Evaluations in Primary Open-Angle Glaucoma: Decision Analytic Modeling Insights
PharmacoEconomics - Open (2020)