Abstract
Purpose
The intra- and postoperative characteristics of two foldable single-piece intraocular lenses (IOL) with identical hydrophilic acrylic material, but different haptic designs (Akreos Adapt® and Akreos Fit®), were compared in combined phacoemulsification and pars plana vitrectomy (PPV).
Methods
This was a prospective, randomized study in patients with simultaneous cataract and vitreoretinal surgery. Group A (n=47 patients) included implantation of Akreos Fit® IOL (two-point haptic) and group B (n=46 patients) implantation of Akreos Adapt® IOL (four-point haptic). All intraoperative modifications of small-incision phacoemulsification and three-port PPV and IOL implantation and centration were documented. At 1 and 2 days and 6 months after surgery, best-corrected visual acuity (BCVA), slit-lamp appearance (including inflammation, IOL centration, capsulorhexis diameter, posterior capsule opacification (PCO), tonometry, and fundus findings were evaluated.
Results
The groups did not differ with respect to age, surgical indications and modifications, intraoperative IOL handling, and centration. At day 2, inflammation and capsulorhexis diameters were similar, but IOL decentration was slightly more frequent with Akreos-Fit® IOLs. Six months after surgery, the rates of PCO, posterior synechiae, and BCVA were similar. Akreos-Fit® had slightly smaller capsulorhexis diameters and slightly more capsular contraction and IOL decentration (P>0.05).
Conclusions
Both of the Akreos IOL are feasible for combined phacoemulsification and PPV. Although similar in intraoperative handling, BCVA, and PCO, IOL centration was slightly better with Akreos-Adapt® than with Akreos-Fit® after combined surgery.
Similar content being viewed by others
Introduction
Combined phacoemulsification and pars plana vitrectomy (PPV) is a procedure often reported to manage coincident cataract and vitreoretinal pathology.1, 2, 3, 4, 5, 6, 7, 8 In general, phacoemulsification with implantation of the intraocular lens (IOL) is the first surgical step. The issue of providing sufficient IOL stability in the capsular bag appears to be of outstanding importance when cataract surgery is combined with PPV. IOL decentration or dislocation may appear intraoperatively as a result of the additional surgical manoeuvres during PPV, or postoperatively, as a result of the increased inflammation and zonular stress from the additional vitrectomy procedure.
As this may also increase the posterior capsule opacification (PCO) rate, provision of accurate IOL stability in the capsular bag is of utmost importance. Small-incision surgery and implantation of foldable IOL have been suggested to reduce postoperative inflammation that may lead to IOL decentration and PCO. In comparison to others, IOL with hydrophilic acrylic material has been shown to exhibit good biocompatibility. Previous studies have shown that hydrophilic acrylic IOL provides accurate stability in the capsular bag.
Two one-piece hydrophilic acrylic IOL with different haptic designs are provided, one with four haptics (Akreos Adapt®) and another with two C-shaped haptics (Akreos fit®). This prospective, randomized study was performed in order to compare these two hydrophilic acrylic Akreos IOLs in patients with combined PPV and phacoemulsification for intraoperative IOL centration and postoperative IOL decentration and dislocation, capsulorhexis diameter, inflammation, posterior synechiae, PCO, and visual outcome.
Patients and methods
Patients were recruited in a continuous cohort at the Ophthalmology Department at St Franziskus Hospital Muenster. Cases with unilateral incipient cataract and simultaneous vitreoretinal pathology were recruited into the study. Patients with previous anterior segment surgery that frequently results in postoperative fibrin formation, for example, perforating ocular trauma, and proliferative diabetic retinopathy were excluded from the study. The investigation was performed in accordance with the Declaration of Helsinki. Informed consent was obtained before the surgery.
Before the operation, the best-corrected visual acuity (BCVA), slit-lamp and ophthalmoscopical appearance, and intraocular pressure (Goldmann applanation tonometry) were documented. Any associated systemic diseases were recorded.
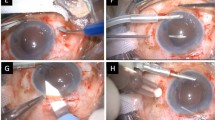
The operations were conducted under general anaesthesia. A standardized endocapsular phacoemulsification surgery was performed in all patients with a limbal incision of 2.5 mm, curvilinear capsulorhexis (CCC) of approximately 5.0 mm, phacoemulsification, irrigation-aspiration for complete removal of the lens cortex. Cells were polished from the posterior lens capsule. The incision was enlarged to 3.2 mm, and an IOL was inserted in the bag.
The patients were randomly assigned to one of the two study groups. The Akreos Adapt® IOL was implanted in one group, and patients in the second group received the Akreos Fit® IOL. Both of the IOLs had hydrophilic acrylic material. The Akreos Adapt® IOL has four haptics. The optic body measures 6.0 mm in diameter. As the diameter of the capsular bag may increase with the axial length of the eye, the total diameter ranges from 10.5 to 11.0 mm, depending on its refractive power. The IOL is inserted with a closed, single-use Hydroport injector system. The Akreos Fit® IOL has C-shaped haptics and an optic diameter of 5.7 mm. The diameter with haptics is 11.5 mm. Insertion is accomplished with forceps.
The limbal incision was temporarily secured with a single 10-0 nylon suture. Then, a standard three-port PPV was performed. Any intraoperative modifications of the surgical technique or complications were documented. The diameter of the capsulorhexis was measured with calipers. IOL handling, especially coverage of the IOL optic with the CCC and centration or dislocation of the IOL during the surgical manoeuvres, was documented. The postoperative medication consisted of dexamethasone and gentamycin eye drops, each five times daily, and atropine eye drops three times daily.
At 2 days and 6 months postoperatively, a visual acuity test, slit-lamp examination, applanation tonometry, and ophthalmoscopy were performed. Cells within the anterior chamber were graded on a scale from 0 to 4+. The diameters of the capsulorhexis were measured. Coverage of the IOL optic with CCC was judged as 360°, partial, or uncovered. The presence of IOL decentration or dislocation, pigment cells or fibrinous membranes on the IOL surface, fibrin formation or silicone oil in the anterior chamber, posterior synechiae, and PCO was documented.
Statistical analysis was performed with the SPSS program (version 10.0). The χ2-test and Student's t-test were applied to determine whether differences between the groups were statistically significant. Parameters such as anterior chamber cells, IOP, and diameter of capulorhexis were analysed with Student's t-test and the Mann–Whitney U-test. P<0.05 was judged as significantly different.
Results
Patient population
There were 93 patients included in the study. In 47 patients, the Akreos Fit® IOL was inserted, and in another 46 the Akreos Adapt® IOL. Gender, visual acuity, and the vitreoretinal pathologies indicating combined PPV did not differ significantly between the two groups (Table 1). The associated systemic diseases found were diabetes mellitus (n=20), systemic arterial hypertension (n=29), rheumatoid arthritis (n=10), and asthma (n=7).
Intraoperative observations and surgical methods
There were no marked differences with respect to the judgment of intraoperative handling and characteristics of the two IOLs (Table 2). In general, both had good intraoperative handling, implantation into the capsular bag was easy, and the IOL was centred spontaneously in the bag. Also, IOL unfolding in the bag was judged to be similar for both IOL types.
The diameters of the capsulorhexis did not differ between the groups and were 4.7±0.45 and 4.65±0.45 mm in the Akreos Fit® IOL and the Akreos Adapt® IOL groups, respectively (P=0.59). The CCC and the IOL were well centred in all eyes operated upon in both groups. In all eyes, the IOL optic was covered with the CCC by 360°. The surgical methods are summarized in Table 2 and did not differ profoundly between groups.
Findings 2 days after surgery
No vision-threatening complications occurred in any of the 93 patients after the surgery. The numbers of inflammatory cells and the frequency of fibrin formation in the anterior chamber did not differ between groups (Table 3). Posterior synechiae were not seen in any of the patients in either group. The mean IOP did not differ significantly between groups, being 18.09±5.86 mmHg in the Akreos Fit® group and 16.22±4.36 mmHg in the Akreos Adapt® group (P=0.123).
The capsulorhexis diameters did not differ between groups and were 4.85±0.31 mm in the Akreos Fit® group and 4.81±0.39 mm in the Akreos Adapt® group (P=0.171). whereas in the Akreos Fit® group of patients, a decentration with partial coverage of the IOL optic with CCC was found in three eyes, the Akreos Adapt® was well centred in all eyes, and CCC covered the IOL optic 360° in all patients. IOL dislocation has not been noted in any of the eyes operated upon.
Findings 6 months after surgery
None of the patients in either group missed the postoperative follow-up visit at 6 months. The diameters of the capsulorhexis did not differ between the Akreos adapt® and Akreos Fit® group of patients (Table 4). Compared with the intraoperative measures, the mean diameters of the capsulorhexis were not significantly changed. Capsular contraction was slightly more common in Akreos FIT® than in the Adapt® group. The frequencies of secondary glaucoma, number of anterior chamber cells, pigment cells, posterior synechiae, and fibrinous membranes did not differ between groups.
Although the capsulorhexis was still well centred in most of the eyes, decentration was evident in few patients in both groups (Table 4). In three additional eyes with Akreos Fit® IOL, IOL decentration was noted. In contrast, well-centred IOLs were documented in the Akreos Adapt® group (P=0.085). PCO was similar for the two lenses (Table 4). The number of Nd : YAG laser capulotomies were not significantly different between the groups.
Compared with the preoperative status, BCVA had significantly (≥2 lines) improved in many patients after the surgery (Table 4). The visual outcome was largely determined by the underlying retinal pathology. The BCVA after 6 months did not differ between the two groups.
Discussion
The present study compared two types of IOLs with identical hydrophilic acrylic material, but with different haptic designs. The data reveal that both types of IOLs are excellent with respect to intraoperative handling and centration in the capsular bag. At 6 months after surgery, PCO rates and IOL centration are encouraging with both IOLs. However, the four-haptic design was slightly better with respect to the prevention of postoperative IOL decentration and capsular contraction than the two-haptic IOL.
The issue of providing accurate IOL stability in the capsular bag appears to be of outstanding importance when cataract surgery is combined with PPV. The IOL decentration or dislocation may appear intraoperatively as a result of the additional surgical stress on the capsular bag and zonular fibres during the vitrectomy. Some surgeons, therefore, recommend that cataract extraction and IOL implantation be performed as a separate procedure 2–4 weeks before the vitrectomy to allow the IOL haptics to be fixed by fibrosis in order to reduce the risk of IOL dislocation.9 Others have not found IOL decentration to be more likely during or after combined surgery.10 Our data show that intraoperative IOL centration was excellent and no decentration occurred with either of the hydrophilic acrylic IOLs.
Decentration of the IOL may occur postoperatively as a result of the increased inflammation and zonular stress from the additional vitrectomy procedure.1, 11, 12, 13 Our findings support previous observations that the incidence of cells and fibrin formation in the anterior chamber is increased in combined surgery compared to what is typically noted after standard phacoemulsification. However, we did not find a difference between the two IOLs used.
In a previous study, the degree of IOL decentration and tilt and the percentage of anterior capsule contraction in eyes with a one-piece acrylic IOL with soft acrylic loops were similar to that noted in eyes with a three-piece acrylic IOL.14 Both of the IOLs used herein were one-piece lenses.
In another report, Hayashi found that among the IOL factors examined, optic material affects most significantly the degree of anterior capsule contraction, whereas the optic design and the haptic material and design are not strongly related to anterior capsule contraction.15 Our findings are not completely in agreement with this. Although the mean capsulorhexis diameters did not differ between the two- and the four-haptic IOLs, there was a tendency towards increased capsular contraction in the two-haptic group. It might be speculated that the slightly improved centration of the Akreos adapt® IOL is related to the slightly larger optic body compared to the Akreos fit® IOL. Conversely, the total diameter of the Akreos fit® is with 11.5 mm slightly larger than the Akreos adapt® IOL with 10.5–11.0 mm.
Ohmi and Uenoyama16 found that postoperative IOL decentration is influenced by asymmetric capsular shrinkage, which is affected by the anterior capsulotomy shape. In our study, a well-centred circular continuous CCC with similar diameter was performed in all eyes, and we only found slight differences in the rate of decentration at 2 days and 6 months after surgery. Our observations demonstrate that the two IOL designs used in the study provide good long-term centration of the capsulorrhexis.
In our study, the Akreos Adapt® IOL with its four-haptic design showed slightly better intraoperative and postoperative centration than the Akreos Fit® IOL, although the differences did not reach the level of statistical significance. It might be speculated that this provides a more accurate IOL fixation in the capsular bag, leading to a constant tension on the zonular fibres.
The observations herein show that the degree of PCO and the frequency of YAG-laser capsulotomies did not differ markedly between the four-haptic IOL (Akreos adapt®) and the two-haptic IOL (Akreos Fit®). The relatively high PCO frequency in both groups might be due to the fact that PC polishing, which is generally performed before PPV, was incomplete or because of the use of SF6-gas or silicone-oil tamponades, which is also known to increase the PCO rate.6
It has been shown that the diverse IOL materials differ with respect to the PCO rates, being relatively low in acrylic IOLs.17 In addition, a sharp posterior edge of the optic has been ascribed to reduce the PCO rate.18 It is noteworthy that the two types of IOLs implanted in this study are made of acrylic material and both of them have sharp posterior optic edges.
In our study, postoperative visual acuity improved by two or more lines in 24 and 25 patients in the Akreos Adapt® and the Akreos Fit® group, respectively. In all cases with poor visual outcome, this correlated with the macular pathology and not with the type of IOL implanted.
References
Koenig SB, Han DP, Mieler WF, Abrams GW, Jaffe GJ, Burton TC . Combined phacoemulsification and pars plana vitrectomy. Arch Ophthalmol 1990; 108: 362–364.
Mamalis N, Teske MP, Kreisler KR, Zimmerman PL, Crandall AS, Olson RJ . Phacoemulsification combined with pars plana vitrectomy. Ophthalmic Surg 1991; 22: 194–198.
Koenig SB, Mieler WF, Han DP, Abrams GW . Combined phacoemulsification, pars plana vitrectomy, and posterior chamber intraocular lens insertion. Arch Ophthalmol 1992; 110: 1101–1104.
Senn P, Schipper I, Perren B . Combined pars plana vitrectomy, phacoemulsification, and intraocular lens implantation in the capsular bag: a comparison to vitrectomy and subsequent cataract surgery as a two-step procedure. Ophthalmic Surg Lasers 1995; 26: 420–428.
Hurley C, Barry P . Combined endocapsular phacoemulsification, pars plana vitrectomy, and intraocular lens implantation. J Cataract Refract Surg 1996; 22: 462–466.
Scharwey K, Pavlovic S, Jacobi KW . (Early posterior capsule fibrosis after combined cataract and vitreoretinal surgery with intraocular air/SF6 gas tamponade). Klin Monatsbl Augenheilkd 1998; 212: 149–153.
Lam DS, Rao SK, Chan WM, Leung AT . Combined phacoemulsification and pars plana vitrectomy. J Cataract Refract Surg 1999; 25: 1309–1311.
Jackson TL, Larsson J, Tanner V, Williamson TH . Combined phaco-emulsification cataract extraction and pars plana vitrectomy without intra-ocular lens implantation. Ophthalmologica 2001; 215: 271–275.
Benson WE, Brown GC, Tasman W, McNamara JA . Extracapsular cataract extraction, posterior chamber lens insertion, and pars plana vitrectomy in one operation. Ophthalmology 1990; 97: 918–921.
Scharwey K, Pavlovic S, Jacobi KW . Combined clear corneal phacoemulsification, vitreoretinal surgery, and intraocular lens implantation. J Cataract Refract Surg 1999; 25: 693–698.
Goodman DF, Stark WJ, Gottsch JD . Complications of cataract extraction with intraocular lens implantation. Ophthalmic Surg 1989; 20: 132–140.
McElvanney AM, Talbot EM . Posterior chamber lens implantation combined with pars plana vitrectomy. J Cataract Refract Surg 1997; 23: 106–110.
Miyake K, Asakura M, Kobayashi H . Effect of intraocular lens fixation on the blood-aqueous barrier. Am J Ophthalmol 1984; 98: 451–455.
Hayashi K, Hayashi H . Comparison of the stability of 1-piece and 3-piece acrylic intraocular lenses in the lens capsule. J Cataract Refract Surg 2005; 31: 337–342.
Hayashi K, Hayashi H . Intraocular lens factors that may affect anterior capsule contraction. Ophthalmology 2005; 112: 286–292.
Ohmi S, Uenoyama K . Decentration associated with asymmetric capsular shrinkage and intraocular lens design in a rabbit model. J Cataract Refract Surg 1995; 21: 293–296.
Hayashi K, Hayashi H, Nakao F, Hayashi F . Changes in posterior capsule opacification after poly(methyl methacrylate), silicone, and acrylic intraocular lens implantation. J Cataract Refract Surg 2001; 27: 817–824.
Nishi O, Nishi K, Wickstrom K . Preventing lens epithelial cell migration using intraocular lenses with sharp rectangular edges. J Cataract Refract Surg 2000; 26: 1543–1549.
Author information
Authors and Affiliations
Corresponding author
Additional information
Relationship disclosure: Supported in part by Bausch & Lomb Surgical
None of the authors has any financial or proprietary interest in the materials or methods mentioned
Rights and permissions
About this article
Cite this article
Mingels, A., Koch, J., Lommatzsch, A. et al. Comparison of two acrylic intraocular lenses with different haptic designs in patients with combined phacoemulsification and pars plana vitrectomy. Eye 21, 1379–1383 (2007). https://doi.org/10.1038/sj.eye.6702446
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702446
Keywords
This article is cited by
-
Effect of intraocular lens material and haptic design on anterior capsule contraction after cataract surgery: A systematic review and meta-analysis
Graefe's Archive for Clinical and Experimental Ophthalmology (2024)
-
Hyperopic shift caused by capsule contraction syndrome after microincision foldable intraocular Lens implantation: case series
BMC Ophthalmology (2019)
-
Effect of number and position of intraocular lens haptics on anterior capsule contraction: a randomized, prospective trial
BMC Ophthalmology (2018)
-
Grundlagen des refraktiven Linsenaustausches
Der Ophthalmologe (2008)