Abstract
Aim
To devise a quantative method for the measurement of the extent of macular subretinal fluid using optical coherence tomography (OCT), and to assess the interobserver and intraobserver agreement for this grading system.
Methods
Observational cohort series. Patients were a cohort who underwent retinal detachment surgery over an 18-month period. All patients had OCT scan at 6 weeks after surgery. The scans were graded by two independent observers experienced in OCT interpretation and each grader was masked to the others findings. Observer 1 then regraded the scans on a later day masked to his previous findings. The interobserver and intraobserver agreement was assessed using weighted Kappa (Kw) statistics.
Results
In all, 116 patients were analysed. Both the intraobserver and interobserver agreement was very high, with Kw being 0.9631 and 0.9070, respectively.
Conclusions
The grading system for assessment of the extent of macular subretinal fluid using OCT appears to have very good reproducibility and repeatability. We propose that this grading system would be clinically useful when applied to pathologies visible on OCT scan of the macula.
Similar content being viewed by others
Introduction
Optical coherence tomography (OCT) is a useful noninvasive tool for detecting macular pathologies, the morphology of which in some cannot be seen on clinical examination.1 Persistent subretinal fluid has been identified by OCT in patients who have undergone successful retinal detachment surgery.2, 3, 4, 5, 6 This fluid often cannot be seen on slit-lamp clinical examination, is not detected by fluorescein angiography and is associated with poor vision and upon its resolution with visual improvement.
Other studies from our group (data in preparation) have determined the incidence, pattern, and duration of persistent subretinal fluid in patients having undergone vitrectomy/gas and scleral buckle procedures for retinal detachment. The results of these studies have raised the question that the extent of subretinal fluid involvement of the macula may also affect the extent to which patients recover following retinal detachment surgery. We have therefore devised a grading system to assess the extent of macular subretinal fluid on OCT and we here present our findings on the reproducibility and repeatability of such a grading system.
Materials and methods
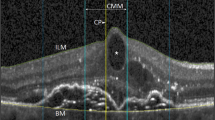
Patients were a cohort of those recruited to other studies undertaken in the vitreoretinal research unit at Moorfields Eye Hospital, the aims of which were to assess the OCT appearance of the macula following retinal detachment surgery. Both the original studies and this further analysis of data had full ethical approval from the local research ethics committee (REC reference numbers CHAD1011, BENS1003, BENS1004) and adhered to the tenets of the Declaration of Helsinki. All patients underwent analysis by STRATUSOCT Model 3000 scanner (Zeiss Humphrey Instruments, Dublin, CA, USA) radial line scan producing 6 × 6 mm scans at 6 weeks following retinal detachment surgery (either scleral buckle or vitrectomy and gas) and had consented for their scans to be used for research. All six scans were examined independently by two observers and the scan with the most extensive involvement of the macula was then assessed according to the grading system we have devised (Figure 1). This was carried out using a clear cellophane overlay marked with eight different graded areas, which was placed on each scan.
The 6 mm scan was divided into three equal areas, each measuring 2 mm in horizontal length. The central 2 mm was termed ‘A’ and the two peripheral areas ‘B’. Area ‘B’ was further subdivided into ‘BP’: area B partial involvement (up to 50% of area B); or ‘BC’: area B complete involvement (over 50% of area B but not involving area A).
If subretinal fluid extended into area ‘A’, one of the subdivision grades of area A was assigned. Up to 25% involvement was graded as marginal involvement and assigned grade ‘AM’ (Figure 1), up to 50% was graded as partial involvement and assigned ‘AP’, up to 75% extensive involvement ‘AE’, and from 75 to 100% of area A total involvement ‘AT’. If none of the 6 mm scan had subretinal fluid, a grade of ‘N’ denoting normal was assigned. If the entire 6 mm scan had subretinal involvement, a grade of ‘T’ denoting total involvement was assigned.
An ophthalmologist experienced in OCT graded all 6-week scans for degree of macular involvement for all patients entered into the study. A second ophthalmologist experienced in OCT independently graded the 6-week OCT scan masked to the findings of the first observer. Both observers were masked to any clinical information on the patients. We then analysed how closely the two gradings matched (interobserver reliability). One of the graders, then at a different time (a week later) and masked to their original grading, regraded the scans, thus allowing assessment of intraobserver reliability.
The principle outcome measure was the percentage agreement between and within observers. We tabulated the percentage agreement, and computed weighted Kappa (Kw) statistics. The weighted Kappa was used due to the ordinal nature of the categories and weights assigned as recommended by Altman are shown in Table 1.7
Kappa-statistic was interpreted according to the recommendations of Landis and Koch,8 whereby a Kappa-value of <0.00 equates to poor strength of agreement, 0.00–0.20 slight agreement, 0.21–0.40 fair agreement, 0.41–0.60 moderate agreement, 0.61–0.80 substantial agreement, and 0.81–1.00 almost perfect agreement. We also present unweighted Kappa values.
Results
The maculas of 116 patients were analysed. The intraobserver agreement on the grading of macular subretinal fluid was very good with a Kw=0.9631 (expected agreement=60.48% and observed agreement=98.54%). The agreement was also very good when unweighted Kappa was used with K=0.8718 (expected agreement=26.04% and observed agreement=90.52%). See Table 2 for tabulated agreement data.
Likewise, the interobserver agreement was very good with a Kw=0.9070 (expected agreement=60.61% and observed agreement=96.34%). The agreement was also very good when unweighted Kappa was used with K=0.8063 (expected agreement=24.35% and observed agreement=85.34%). See Table 3 for tabulated agreement data.
Discussion
The simple grading system we have devised has been shown to have ‘almost perfect’ agreement in terms of its interobserver and intraobserver variability. To our knowledge, no other reports to date have attempted to grade the extent of macular subretinal fluid on OCT.
We considered a number of variables when producing the grading system, including whether area as opposed to cross-sectional length of the macula involved should be graded, and whether the 6 × 6 mm radial line scan was appropriate. We also considered the inherent problems of fixation, as microsaccades may alter alignment between the six scans, hence the foveola may not be centred.
Grading area as opposed to cross-sectional length of macula involved may have some advantage as it could take account of the height of subretinal fluid (although from our previous study height of subfoveal fluid did not affect visual outcome; data in preparation). However, determining the area involved would require exporting all six raw data scans for each patient for analysis as direct analysis is not possible within the STRATUSOCT. This was considered to be too lengthy a procedure to be of practical use by researchers.
The ‘radial line’ scanning mode of the OCT, while taking a few seconds more than the ‘fast macula scan’, provides greater resolution of image (512 A scans vs 128 A scans),9 and was chosen so that small areas of subretinal fluid would not be missed. We acknowledge however that 6 × 6 radial line scan examines a very limited area of the entire macula; however, as each line scan is evenly spread at a 60° angle, we considered that the scanning option represented a fair compromise as to what is practical in the clinical setting. With regard to fixation, scans were either repeated until good fixation was obtained or the external fixator light on the OCT was used to obtain optimum fixation in all patients.
The grades of ‘A’ and ‘B’ were assigned as we felt that the central area of the macula ‘A’ should be distinguished because of the increased density of photoreceptors and importance in central vision. While this central 2 mm does not correspond anatomically to any demarcation, it was deemed a reasonable and practical area.
The weighted Kappa is an accepted method for assessing the agreement of ordinal data, however, the weights assigned are, in all cases, relatively arbitrary.10 We therefore have included also the unweighted Kappa results. Weights were assigned according to the recommendation by Altman (Table 1).7 The grading system here described for assessment of extent of subretinal fluid involvement of the macula provides almost perfect agreement in terms of interobserver and intraobserver variability as defined by Landis and Koch.8
A highly reproducible and repeatable grading system has both research and clinical implications. The same grading system could be applied to other pathologies such as drusen, epiretinal membrane and RPE change, although the pathologies it may be applied to are limited by the resolution of the current STRATUSOCT (<8 μm axial resolution).9 It also permits assessment of extent of disease processes over time and may be used to assess the natural history of pathology and the response to treatment and therefore may be a useful tool in assessing prognosis and in clinical trials.
References
Puliafito CA, Hee MR, Lin CP, Reichel E, Schuman JS, Duker JS et al. Imaging of macular diseases with optical coherence tomography. Ophthalmology 1995; 102: 217–229.
Wolfensberger TJ, Gonvers M . Optical coherence tomography in the evaluation of incomplete visual acuity recovery after macula-off retinal detachments. Graefes Arch Clin Exp Ophthalmol 2002; 240: 85–89.
Hagimura N, Iida T, Suto K, Kishi S . Persistent foveal retinal detachment after successful rhegmatogenous retinal detachment surgery. Am J Ophthalmol 2002; 133: 516–520.
Kaga T, Fonseca RA, Dantas MA, Yannuzzi LA, Spaide RF . Optical coherence tomography of bleb-like subretinal lesions after retinal reattachment surgery. Am J Ophthalmol 2001; 132: 120–121.
Theodossiadis PG, Georgalas IG, Emfietzoglou J, Kyriaki TE, Pantelia E, Gogas PS et al. Optical coherence tomography findings in the macula after treatment of rhegmatogonous retinal detachments with spared macula preoperatively. Retina 2003; 23: 69–75.
Wolfensberger TJ . Foveal reattachment after macula-off retinal detachment occurs faster after vitrectomy than after buckle surgery. Ophthalmology 2004; 111: 1340–1343.
Altman DG . Practical Statistics for Medical Research. Chapman & Hall/CRC Press: London/Boca Raton, 1999, pp 406–407.
Landis RJ, Koch GG . The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–174.
ZEISS. STRATUSOCT User Manual PN 55153-1 REV. A 12/02, 2002.
Brennan P, Silman A . Statistical methods for assessing observer variability in clinical measures. BMJ 1992; 304: 1491–1494.
Author information
Authors and Affiliations
Corresponding author
Additional information
Financial support provided by PfizerNo conflicting relationship exists for any of the above authors
Rights and permissions
About this article
Cite this article
Benson, S., Schlottmann, P., Bunce, C. et al. Assessment of the reproducibility and repeatability of a method of grading macular subretinal fluid using optical coherence tomography. Eye 20, 1030–1033 (2006). https://doi.org/10.1038/sj.eye.6702073
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702073