Abstract
Purpose
To assess the validity and repeatability of partial coherence interferometry (IOLMaster) and A-scan ultrasound measurement of axial length (AL) in children.
Methods
A prospective comparison of AL measurement made by the IOLMaster optical instrument (Carl Zeiss) and ultrasound A-scan (Alcon) was performed. A total of 20 children (11 male, nine female) were recruited into the study; the mean age of the sample was 11.4 years (range 6.2–15.8). Inclusion criteria comprised individuals <16 years, with no ocular pathology and no previous eye operations or allergy to topical anaesthetics. All measurements were performed by a single examiner.
Results
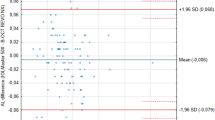
Data on validity show that, on average, a small measurement difference existed between these groups with the IOLMaster being 0.017 mm greater than A-scan ultrasonography. The 95% confidence interval for this difference encompasses zero, demonstrating that no significant systematic bias exists between the two-measurement techniques. Overall, IOLMaster reliability exceeded that of A-scan. This is evidenced primarily by the spread of the paired test–retest difference for A-scan compared to IOLMaster. The mean test–retest difference for A-scan was considerably larger than IOLMaster at 0.042 and 0.004 mm, respectively.
Conclusion
The results show that IOLMaster was more accurate and reproducible than the contact ultrasonographic technique when used in children. Such results indicate that IOLMaster may be a useful tool in studies of eye growth and refractive development in children.
Similar content being viewed by others
Introduction
A-scan ultrasound biometry (ASU) is the conventional method for measurement of axial length (AL). The major drawback of ASU is that contact between the ultrasound probe and the cornea requires local anaesthetic use and the contact may cause corneal abrasion or infection.1
Studies based on preoperative and postoperative biometry demonstrated that 54% of the errors in predicted refraction after implantation of an intraocular lens (IOL) could be attributed to AL measurement errors.2 In AL measurement, an error of 100 μm corresponds to an error in postoperative refraction of ±0.28 D.2, 3
The IOLMaster™ (Carl Zeiss Meditec, Dublin, CA, USA) provides an alternative technique for measurement of a number of ocular parameters, based on partial coherent interferometry (PCI). This instrument is primarily designed for calculating the IOL power, by measurement of AL and corneal curvature. IOLMaster can also measure anterior chamber depth. It has several advantages over the ASU for measurement of ocular parameters; (1) it uses a noncontact method therefore avoiding anaesthesia, (2) transmission of infection is minimised due to noncontact methodology and (3) the method precludes corneal indentation, an additional source of AL measurement error.
Several validity and repeatability studies have been conducted in adults that have compared PCI with ASU.1, 4, 5, 6 These studies have shown that PCI measurements of AL are precise and repeatable. However, to our knowledge, there are scant data describing the validity and repeatability of PCI measurements in smaller eyes, such as in children. This is important, as findings of previous studies cannot be generalised to this group. The aim of this study was to assess the validity and repeatability of PCI (IOLMaster) and ASU measurements of AL in children.
Materials and methods
Study design
A prospective case series study design was used. Ethical approval was obtained from the South West Local Research Ethics Committee and informed consent from the child and/or parents (as appropriate) was obtained before commencing the test.
Recruitment
Relatives of individuals attending Bristol Eye Hospital were approached. Inclusion criteria included individuals <16 years, with no ocular pathology and no previous eye operations or allergy to topical anaesthesia. Exclusion criteria included nystagmus, amblyopia or any other ocular pathology likely to affect visual function.
Measurements
AL measurements were made in both eyes using PCI and ASU by a single examiner (HMH).
For PCI, the child's head was adjusted and the child was asked to fixate on the red alignment beam. The reflection of the alignment light was placed within the sighting circle to achieve a measurement. Three measurements were taken for each eye and the average was used for analysis. A signal-to-noise ratio (SNR) above 2 was required for the measurements to be valid. AL was then measured by ASU. One drop of topical anaesthetic (Benoxinate hydrochloride 0.4%) was instilled in each eye 2 min before measurements were taken. The child was asked to fixate on a target and a hand-held probe was applied to the cornea. Special care was taken to apply minimal pressure to the cornea.
Statistical analysis
One eye was randomly selected from each subject for statistical analysis. Assessment of both validity and reliability were based on the analysis of paired measurements at the level of individual subjects. For validity, or measurement accuracy, paired measures comprised those of the established reference (or ‘gold’) standard, ASU and those of the candidate technique, PCI. Quantification of reliability (repeatability), or measurement precision, was based on paired repeated measures made on two separate test sessions.
Two identical statistical approaches were used to quantify both validity and reliability. In the first, attention was focused on score differences for paired measures. These were examined for evidence of systematic bias between the two measurements (mean difference and corresponding 95% confidence interval for the mean paired difference) and for the extent of difference within individual pairs. Quantification of the spread of differences, using the standard deviation (SD) of the mean difference, was used to calculate the ‘limits of agreement’ (±1.96 × SD), within which 95% of the sample differences occur. In the context of validity, comparison of the limits of agreement with an accepted clinically significant difference provides a guide as to whether the candidate technique may be clinically acceptable.
The second approach to reliability assessment involved calculation of the quadratic weighted kappa statistic (kw). This chance-corrected measure of agreement weights degrees of discrepancy according to the square of the difference between the (paired) measurements.7 There are no universally applicable standard values for this statistic that represent adequate reliability, but to aid presentation the following convention is followed here: ICC <0.20 ‘slight agreement’; 0.21–0.40 ‘fair agreement’; 0.41–0.60 ‘moderate agreement’; 0.61–0.80 ‘substantial agreement’; and above 0.80 ‘almost perfect agreement’.6 Use of kw is preferable to the usual (Pearson) correlation coefficient since the latter measures association rather than agreement.
Results
A total of 20 individuals (11 male, nine female) were recruited into the study. The mean age of the sample was 11.4 years (range 6.2–15.8). Of the eyes randomly selected for analysis (10 right: 10 left), the average AL at the first measurement session using PCI was 22.50 mm (range 21.24–23.65). A frequency distribution of sample ALs is given in Figure 1.
For repeat testing the distribution of inter-test intervals was positively skewed with a median intertest interval of 18 days and range extending from 4 to 98 days. Summary data on validity are given in Table 1. These data show that, on average, a small measurement difference existed between these groups with the PCI 0.017 mm greater than ASU. The 95% confidence interval for this difference encompassed zero, demonstrating that no significant systematic bias existed between the two measurement techniques. The limits of agreement for this comparison were ±0.21 mm. The relative variability between the techniques was 8.7%.
Summary data on reliability of both measurement techniques are provided in Table 2. Overall, PCT reliability exceeded that of ASU. This is evidenced primarily by greater spread of paired test–retest differences for ASU than PCT: 95% limits of agreement were ±0.23 and ±0.04 mm for ASU and PCT, respectively. Approximately equal spread of paired differences across the sample range for both techniques strongly suggests that reliability was independent of AL within the sample range, as found for validity data. In spite of this reliability difference between techniques, the measurements of both exhibit high precision, with relative variability levels below 10% and ‘almost perfect’ levels of agreement between first and second measurements by Kw. Also, neither technique showed evidence of systematic bias, as evidenced by 95% confidence interval encompassing zero. Mean test–retest difference for ASU was considerably larger than PCT at 0.042 and 0.004 mm, respectively.
When considering a candidate measurement technique, it is appropriate to consider the validity of the technique alongside the established method of measurement. In this context, it should be noted that the validity of PCT exceeded the reliability of ASU.
Discussion
In this study, we compared PCI with ASU in children and found almost perfect agreement between the two devices. Our data showed a trend whereby measurement of AL by PCI was longer than ASU, by 0.017 mm (95% confidence interval −0.030–0.065), although this difference was not statistically significant. This finding is consistent with a previous study on healthy eyes of 52 adult subjects aged 18–40 years that found no significant difference in AL between the two devices.4 However, this finding is not in agreement with a number of other studies that have been undertaken on adults. For example, Goyal et al8 found that AL obtained by PCI was significantly higher than that obtained by ASU. Haigis et al9 estimated optically measured AL to be about 0.3 mm longer than ASU. Kielhorn et al10, 11 found that AL was a mean of 0.96 mm longer when the IOLMaster was used.
In contrast to the above studies, Lam et al12 found that IOLMaster measurements of AL were slightly lower than those measured by ASU, though this did not reach statistical significance. It therefore appears that the literature on the relationship between PCI and A-scan is not completely concordant.
In terms of use to establish power of an IOL, studies on ASU, showed that an error of 200 μm in AL measurement would result in a postoperative refractive error of 0.56 D.13 One possible reason for the longer AL measurements by PCI is that ultrasound and light may be reflected from different layers in retina, with ultrasound being reflected from the inner limiting membrane and wavelengths used in PCI being reflected from the retinal pigment epithelium. If correct, this would lead to the expectation that AL measurements made by PCI would be marginally longer than those made by ASU. It is also possible that the applanation technique used during AL measurement by ASU indents the cornea, resulting in shorter AL measurements.
Our study has demonstrated that the reliability of PCI is higher than ASU (Table 2), with the mean test–retest difference for A-scan being considerably larger than PCI at 0.042 and 0.004 mm, respectively. This finding is consistent with that of Vogel et al,1 who found that using IOLMaster for AL measurement was highly reliable. Rajan et al14 compared the accuracy of PCI to ASU for calculation of IOL power and used the mean absolute error of AL difference to assess the repeatability of both devices. Reported values were 0.13 mm for the PCI group and 0.19 mm for the ASU group. Carkeet et al15 also found that IOLMaster has better repeatability compared to ASU when used to measure AL in children.
In summary, our study has shown that the IOLMaster is both repeatable and accurate in measuring the AL in children. As an examination technique, PCI has a number of clinical advantages, offering a high degree of comfort for the patient, avoidance of topical anaesthetic and reduced risk of infection due to the noncontact technique. However, PCI does require patient co-operation and may not be a viable option in young children. These findings suggest that the use of PCI in measuring AL in children is a safe and reliable technique. This technique, therefore, appears to have both a clinical utility and may also be useful in research environments, such as in congenital glaucoma,16 as well as in studies of eye growth and refractive development in children.
References
Vogel A, Dick HB, Krummenauer F . Reproducibility of optical biometry using partial coherence interferometry: intraobserver and iterobserver reliability. J Cataract Refract Surg 2001; 27: 1961–1968.
Olsen T . Sources of error in intraocular lens power calculation. J Cataract Refract Surg 1992; 18: 125–129.
Olsen T . Theoretical approach to intraocular lens calculation using Gaussian optics. J Cataract Refract Surg 1987; 13: 141–145.
Santodomingo-Rubido J, Mallen EA, Gilmartin B, Wolffsohn JS . A new non-contact optical optical device for ocular biometry. Br J Ophthalmol 2002; 86: 458–462.
Connors III R, Boseman III P, Olson RJ . Accuracy and reproducibility of biometry using partial coherence interferometry. J Cataract Refract Surg 2002; 28: 235–238.
Nemeth J, Fekete O, Pesztenlehre N . Optical and ultrasound measurement of axial length and anterior chamber depth for intraocular lens power calculation. J Cataract Refract Surg 2003; 29: 85–88.
Streiner D, Norman G . Health Measurement Scales. Oxford Medical Publications: Oxford, 1995.
Goyal R, North RV, Morgan JE . Comparison of laser interferometry and ultrasound A-scan in the measurement of axial length. Acta Ophthalmol 2003; 81: 331–335.
Haigis W, Lege B, Miller N, Schneider B . Comparison of immersion ultrasound biometr and partial coherence interferometry for intraocular lens calculation according to Haigis. Graefes Arch Clin Exp Ophthalmol 2000; 238: 765–773.
Verhulst E, Vrijghem JC . Accuracy of intraocular lens power calculations using the Zeiss IOL master. A prospective study. Bull Soc Belge Ophthalmol 2001; 281: 61–65.
Kielhorn I, Rajan MS, Tesha PM, Subryan VR, Bell JA . Clinical assessment of the Zeiss IOLMaster. J Cataract Refract Surg 2003; 29: 518–522.
Lam AK, Chan R, Pang PC . The repeatability and accuracy of axial length and anterior chamber depth measurements from the IOLMaster(TM). Ophthalmic Physiol Opt 2001; 21: 477–483.
Olsen T, Nielsen PJ . Immersion vs contact technique in the measurement of axial length by ultrasound. Acta Ophthalmol 1989; 67: 101–102.
Rajan MS, Keilhorna I, Bell JA . Partial coherence laser interferometry vs conventional ultrasound biometry in intraocular lens power calculation. Eye 2002; 16: 552.
Carkeet A, Saw SM, Gazzard G, Tang W, Tan DT . Repeatability of IOLMaster biometry in children. Optom Vis Sci 2004; 81: 829–834.
Kiefer G, Schwenn O, Grehn F . Correlation of postoperative axial length growth and intraocular pressure in congenital glaucoma—a retrospective study in Trabeculotomy and goniotomy. Graefes Arch Clin Exp Ophthalmol 2001; 239: 893–899.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors had no proprietary interests or research funding for the production of this work
Rights and permissions
About this article
Cite this article
Hussin, H., Spry, P., Majid, M. et al. Reliability and validity of the partial coherence interferometry for measurement of ocular axial length in children. Eye 20, 1021–1024 (2006). https://doi.org/10.1038/sj.eye.6702069
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702069
Keywords
This article is cited by
-
Effect of orthokeratology on precision and agreement assessment of a new swept-source optical coherence tomography biometer
Eye and Vision (2020)
-
Myopia
Nature Reviews Disease Primers (2020)
-
Small incision lenticule extraction (SMILE) lenticule thickness readout compared to change in axial length measurements with the IOLMaster
Graefe's Archive for Clinical and Experimental Ophthalmology (2020)
-
Comparison of anterior segment parameters and axial lengths of myopic, emmetropic, and hyperopic children
International Ophthalmology (2019)
-
Circumferential silicone sponge scleral buckling induced axial length changes: case series and comparison to literature
International Journal of Retina and Vitreous (2017)