Abstract
Aim
To assess the effect of standard power vs low power transpupillary thermotherapy (TTT) in patients with active subfoveal choroidal neovascularization secondary to age-related macular degeneration ineligible for photodynamic therapy (PDT) by original treatment of age-related macular degeneration with photodynamic therapy (TAP) study group recommendations.
Methods
Retrospective review of 79 patients with active predominantly occult subfoveal choroidal neovascularization or predominantly classic subfoveal choroidal neovascularization but Snellen visual acuity <20/200. All patients were treated with TTT administered via a Mainster wide field fundus contact lens with a retinal power/diameter coefficient of 248 mW/mm in the standard power (n=27) and 181 mW/mm in the low power group (n=52). The primary outcome was stabilization (<1 Snellen line change) or improvement (two or more Snellen lines) in visual acuity. Clinical and fluorescein angiographic resolution of overlying exudation was documented.
Results
At 24 month follow-up, 17 patients (63%) in the standard power and 36 patients (69%) in the low power group achieved stable or improved vision. Improved vision (mean three lines) was observed in 22% of the standard power and 23% of the low power group. Overlying exudation was reduced clinically with minimal or no leakage on fluorescein angiogram in 85% of standard power and 90% of low power group. Subgroup analysis in the low power group demonstrated a visual benefit in patients with subfoveal lesions, which had any classic component.
Conclusions
Low power TTT is as effective as standard power in stabilizing or improving vision and reducing overlying exudation in patients with active subfoveal choroidal neovascularization ineligible for PDT.
Similar content being viewed by others
Introduction
Age-related macular degeneration (AMD) is responsible for progressive central visual loss, which affects the majority of our activities of daily living. The hallmark of neovascular AMD is the development of choroidal neovascularization (CNV), which ultimately leads to the destruction of the macular photoreceptors and is responsible for severe central visual loss, accounting for 88% of AMD patients who are blind.1 The recent Beaver Dam Eye study2 reported the cumulative incidence of neovascular AMD in at least one eye as 1.4% in the aged population. At present the only proven treatments, conventional argon laser photocoagulation3, 4, 5 and photodynamic therapy (PDT),6, 7 have limitations. The immediate profound central scotoma following conventional laser for subfoveal CNV has rendered it an unacceptable clinical treatment modality. The Treatment of age-related macular degeneration with photodynamic therapy (TAP) study group6, 7 demonstrated stabilization or improved vision following PDT with verteporfin in patients with predominantly classic subfoveal CNV lesions and recent findings from the Verteporfin in Photodynamic Therapy (VIP) study8 demonstrated a visual benefit in small purely occult CNV lesions. Patient eligibility for PDT remains limited by visual acuity requirements, angiographic characteristics, and lesion size.
The majority of patients presenting with new onset active subfoveal CNV have occult or minimally classic lesions and are therefore not eligible for current proven treatments. Furthermore, the natural history of these lesions is a progressive increase in the lesion size and concomitant visual loss. Transpupillary thermotherapy (TTT) has been used for the treatment of small posterior choroidal melanomas,9 retinoblastoma,10 and choroidal haemangiomas11 since the mid 1990s. Recent interest has focused on the potential therapeutic effect of TTT on subfoveal CNV secondary to AMD following observations by Reichel et al12 in 1999 and more recent publications.13, 14, 15 TTT has been shown to stabilize vision and reduce overlying exudation in both classic and occult subfoveal CNV.12, 13, 14, 15, 16 The exact treatment parameters have not yet been elucidated. Based on previous clinical experience, Goldmann type contact lens is most commonly used generating a power retinal/diameter coefficient of 248 mW/mm. Despite being usually well-tolerated, concerns exist about the risk of a retinal pigment epithelium (RPE) tear, macular ischaemia, and possible longer term RPE atrophy particularly with repeat treatments.
During the time parameters of this study PDT in our unit was restricted to those patients whose visual acuity and lesion characteristics conform to the TAP criteria.6, 7 This study aimed to assess the outcomes of low power TTT compared to a previously treated group of standard power TTT in patients with subfoveal CNV not eligible for PDT.
Materials and methods
Retrospective review of patients with a recent diagnosis of subfoveal CNV secondary to AMD, ineligible for PDT according to the TAP protocol, and who received TTT was performed. Patients with predominantly classic CNV had visual acuity <20/200. Patients were not offered treatment if they clinically had a large amount of serous subretinal fluid overlying the neovascular complex, cystic retinal changes predisposing to macular hole development, or greater than 50% of the CNV complex comprising of haemorrhage.
At the initial and each subsequent clinic visit, patients underwent measurement of best-corrected visual acuity on either a 2 m logMAR chart or a Snellen chart and detailed macular examination with high magnification slit lamp biomicroscopy. Visual acuity tested on a 2 m logMAR chart was converted to Snellen equivalent. Colour fundus photography and fluorescein angiography (sodium fluorescein 5 ml, 10%) were performed at each visit. The lesions were classified based on fluorescein characteristics. Baseline lesion characteristics including presence of haemorrhage, fibrosis, and retinal pigment epithelium (RPE) changes were documented (Table 1).
TTT was delivered using a slit lamp mounted modified infrared diode laser (Iridex, Mountain View, California) at 810 nm via a hand held contact lens (Mainster Wide Field) with a magnification power of 1.5 using a 2.0 or 3.0 mm slit lamp spot. The initial group of 29 patients were treated with power settings of 740–1100 mW generating a power retinal/diameter coefficient of 248 mW/mm. In the subsequent low power group (n=52), power settings of 530–800 mW were employed generating a power retinal/diameter coefficient of 181 mW/mm. Duration of treatment was for 1 min with no visible retinal whitening. Lesions with linear dimensions greater than 4.5 mm were treated with overlapping spots.
Follow-up visits were planned at 6 weeks and thereafter 3 monthly. The primary outcome was stabilization of vision, defined as less than one Snellen line change. Improved visual acuity was defined as an increase in Snellen visual acuity of two or more lines from baseline. Worse visual acuity was defined as loss of two or more Snellen lines of visual acuity from baseline. We assessed avoidance of moderate (<3 line) and severe (<6 line) visual loss. Clinical and fluorescein angiogram outcomes were documented (Table 2). The mean of the figures obtained after calculation of the logarithm of the minimal angle of resolution (Snellen visual acuity) was converted to the antilog in order to obtain the average visual acuity. Visual acuity outcomes were compared by conversion to logMAR using a paired two-tailed Student's t-test. Visual outcomes between subgroups of differing lesion size and differing lesion component were compared using a χ2 test (Table 3).
Results
In all, 79 eyes of 79 patients who received TTT were included in the study. Baseline characteristics including sex, age, and presenting visual acuity were similar in both groups. (Table 1) Follow up in the standard power group (mean 25 months) was longer than in the low power group (mean 21 months). Mean number of treatments per patient was 1.2 (range 1–3) in both.
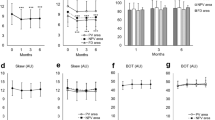
All 79 CNV lesions were subfoveal. In the standard power group, 74% were pure occult and 26% were minimally classic. In the low power group, 73% were pure occult, 15% were minimally classic, and 12% were predominantly classic. There was haemorrhage present in 56% of the lesions in both groups at presentation. There was no significant difference in mean visual acuity before and after TTT in the standard power group (20/180 vs 20/220) or the low power group (20/200 vs 20/190) (Table 2).
In total, 63% (standard power) and 69% (low power) achieved the primary outcome of stable or improved vision, respectively. Of those, 22% (standard power) and 23% (low power) had improved vision by two or more lines (mean three lines). Patients in the standard power group (37%) had worsened vision of two or more lines as compared to patients in the low power group (31%). In total, 67% (standard power) vs 73% (low power) avoided moderate visual loss and 93 vs 94% avoided severe visual loss, respectively.
Two patients in the standard power group and three patients in the low power group lost more than six lines of vision. Massive exudation with the development of a cystoid macular hole was responsible for vision loss in one patient in the standard power group and in two patients in the low power group. One patient in each group lost six or more lines of vision due to significant central RPE atrophy.
Subgroup analysis of the low power group (Table 3) demonstrated that patients with any classic component in their lesion had stable or improved vision compared to those with pure occult lesions. No significant difference in visual outcomes was noted for smaller (<3600 μm) or larger lesion (≥3600 μm) subgroups.
On fluorescein angiogram, minimal or no leakage was observed in 85% of the standard power and 90% of the low power TTT-treated eyes at latest follow-up. During the procedure, no patient reported any discomfort and no whitening of the lesion was noted in any case. No patient developed an RPE tear following treatment. No serious adverse event occurred during or immediately after treatment in any patient.
Discussion
The most important finding from this study is that visual acuity was stable or improved in 69% of patients following low power TTT, which compares favourably to standard power TTT, PDT, and natural history outcomes. Reichel et al12 in 1999 first showed that TTT for AMD reduced the amount of subretinal fluid overlying a CNV complex in 94% and appeared to stabilize or improve visual acuity in 75%. Other TTT trials have demonstrated angiographic membrane closure following standard power TTT for CNV in 78%13 with stabilization of visual acuity in 62.5–86%.12, 13, 14, 15, 16 The exact optimal treatment parameters are unknown; the initial 29 patients in this series were treated based on previous clinical experience. Power settings of 720–1100 mW were used with a Mainster wide field lens to achieve a power/retinal diameter coefficient of approximately 248 mW/mm. Thach et al15 more recently demonstrated that large spot size TTT is effective in stabilizing or improving visual acuity in 71% of a series of 69 patients with predominantly occult membranes. The power used in this series ranged from 600 to 1000 mW generating a power/retinal diameter coefficient of between approximately 169 and 266 mW/mm. In our series of 52 patients, lower power (530–800 mW, power/retinal diameter coefficient 181 mW/mm) TTT achieved stabilization or improvement in vision (69%) and reduction in subretinal exudation (90%) overlying the CNV complex comparable to the standard power group and similar to previously reported data.
In all, 73% of patients in the low power group avoided moderate visual loss and 94% avoided severe visual loss which compares favourably with the outcomes from the TAP6, 7 and VIP8 studies. The 24-month outcomes of the VIP study group8 demonstrated stable (no change) or improved vision (≥1 line) in only 28% of verteporfin and 20% of placebo group with pure occult subfoveal CNV. In addition, vision decreased (≥1 line) in 72% of verteporfin-treated and in 80% of the placebo-treated group in the VIP study. In our low power group, only 16 patients (31%) had reduced visual acuity of two or more Snellen lines. Subgroup VIP study analysis suggests PDT is of greater benefit to patients with smaller lesions (four disc areas or less) and lower levels of visual acuity.8 In our low power group, no significant difference in visual outcomes was demonstrated between patients with smaller (<3600 μm) or larger (≥3600 μm) lesions (Table 3), suggesting TTT as effective for both. Predominantly, occult neovascular membranes are often large and 17 (33%) of the lesions in our low power group had a greatest linear dimension of at least 3600 μm (equivalent to 4 MPS disc areas). The Mainster wide field lens, which generates a large retinal spot size, permits total coverage of these large lesions. As PDT has not been of any proven benefit for large occult lesions,8 TTT offers a therapeutic option for these patients.
Our visual acuity and exudative outcomes following low power TTT also compare favourably with the natural course of occult subfoveal CNV. One study17 demonstrated that, after 9–12 months of follow-up, 32% of occult lesions more than double their size, the median loss in visual acuity was 2.5 lines and approximately 50% of pure occult lesions developed classic CNV.
Subgroup analysis of the low power group showed that patients with any classic component had stable or improved vision (P<0.03) following TTT compared to patients with purely occult CNV (Table 3). Neither the TAP6, 7nor VIP8 study demonstrated any benefit with PDT for patients with minimally classic lesions or predominantly classic lesions but vision less than 20/200. Unfortunately, the majority of patients with subfoveal CNV have pure occult or minimally classic leakage and often larger lesions. This study has shown a treatment benefit following low power TTT for this large patient population that are ineligible to conventional forms of therapy.
TTT was well tolerated by patients in both groups with no retinal whitening observed and no pain reported by any patient during the procedure. Following treatment, three patients developed increased exudation and angiographic leakage with a cystoid macular hole. No predisposing factors were evident in these patients, thus it is unclear if these effects were directly related to the treatment. Overall, 26 patients between both groups lost more than two lines of Snellen visual acuity, despite a marked improvement in associated overlying exudation in 20 of these eyes.
The main risk with TTT identified in the short term is the development of an RPE tear. Following standard power TTT, the rate of RPE tears has varied from 0% to as high as 8%.12, 13, 14, 15, 16, 18 No patient developed an RPE tear in the standard or lower power groups. Benner et al19 recently reported two cases of macular infarction following TTT in a case series of 107 patients (1.9%), however, both these patients had possible predisposing factors; a pre-existing laser scar and an area of geographic atrophy. None of the patients in our series developed this problem. This low rate of adverse effects also compares well with the documented complication rate following PDT. The rate of acute severe vision loss after PDT (four or more lines on a logMAR chart within 7 days of treatment) was reported as 4.4% in the VIP study.8 None of the patients in our series reported a severe visual loss following initial or any repeat treatment. The long-term potential damaging effects of PDT on RPE cells, particularly in patients requiring multiple treatments, may become a limiting factor in its use.
In practice, TTT is a less invasive form of treatment than PDT for the patient and is not as cost prohibitive. TTT is a straightforward procedure that can easily be carried out in a short time in an outpatient clinic. No medication is transfused during TTT, unlike during PDT, lessening the potential for adverse effects or patient unsuitability. The cost of TTT is restricted to the initial purchase of the portable diode laser and no other specialized equipment is required, in comparison to the administration of PDT. The frequency of repeat treatments after TTT is low as a reduction in exudation is achieved in the majority of cases. The majority of patients in the low power group (77%) required one treatment only. This favourably compares with the repeat treatment rate following PDT particularly in the first year; the average number of treatments during the first year of the TAP study was 3.7.6
Data interpretation from this study has limitations as it is a retrospective review, Snellen and not logMAR visual acuity charts were used and follow up is relatively short. Thus, direct comparison to prospective, randomised studies such as the TAP and VIP cannot be made. However, it does provide further supporting evidence for the role of low power TTT in predominantly occult CNV and for those patients with predominantly classic subfoveal CNV but with lower levels of vision rendering them ineligible for PDT. Proof of a therapeutic benefit is being assessed by a prospective randomised trial, the TTT4CNV trial currently underway.
References
Ferris III FL, Fine SL, Hyman L . Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol 1984; 102: 1640–1642.
Klein R, Klein BE, Tomany SC, Meuer SM, Huang GH . Ten-year incidence and progression of age-related maculopathy: The Beaver Dam Eye Study. Ophthalmology 2002; 109 (10): 1767–1779.
Macular Photocoagulation Study Group. Argon laser photocoagulation for neovascular maculopathy. Five year results from randomized clinical trials. Arch Ophthalmol 1991; 109: 1109–1114.
Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration: results of a randomised clinical trial. Arch Ophthalmol 1991; 109: 1242–1257.
Macular Photocoagulation Study Group. Laser photocoagulation for juxtafoveal choroidal neovascularization. Five year results from randomized clinical trials. Arch Ophthalmol 1994; 112: 500–509.
Treatment of Age-Related Macular Degeneration With Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials-TAP report 1. Arch Ophthalmol 1999; 117: 1329–1345.
Bressler NM, Treatment of Age-Related Macular Degeneration With Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two year results of 2 randomized clinical trials-TAP report 2. Arch Ophthalmol 2001; 119: 198–207.
Verteporfin in photodynamic therapy (VIP) study group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization— verteporfin in photodynamic therapy report 2. Am J Ophthalmol 2001; 131: 541–560.
Shields CL, Shields JA, DePotter P, Kheterpal S . Transpupillary thermotherapy in the management of choroidal melanoma. Ophthalmology 1996; 103: 1642–1650.
Murphree AL, Villablanca JG, Deegan III WF, Sato JK, Malogolowkin M, Fisher A et al. Chemotherapy plus local treatment in the management of intraocular retinoblastoma. Arch Ophthalmol 1996; 114: 1348–1356.
Garcia-Aruma J, Ramsay LS, Guraya BC . Transpupillary thermotherapy for circumscribed choroidal haemangiomas. Ophthalmology 2000; 107: 351–356.
Reichel E, Berrocal AM, Ip M, Kroll AJ, Desai V, Duker JS et al. Transpupillary thermotherapy of occult subfoveal choroidal neovascularization in patients with age-related macular degeneration. Ophthalmology 1999; 106: 1908–1914.
Newsom RSB, Mc Alister JC, Saeed M, McHugh JDA . Transpupillary thermotherapy (TTT) for treatment of choroidal neovascularization. Br J Ophth 2001; 85: 173–178.
Friberg TR, Pandya A, Nazari K . Transpupillary thermotherapy (TTT) for age-related macular degeneration. Semin Ophthamlmol 2001; 16: 70–80.
Thach AB, Sipperley JO, Dugel PU, Sneed SR, Park DW, Cornelius J . Large-spot size transpupillary thermotherapy for the treatment of occult choroidal neovascularization associated with age-related macular degeneration. Arch Ophthalmol 2003; 121: 817–820.
Kim JE, Perkins SL, Schwiesow T, Connor Jr TB, Han DP . Transpupillary thermotherapy of occult choroidal neovascularization in age-related macular degeneration. Semin Ophthalmol 2001; 16: 86–89.
Stevens TS, Bressler NM, Maguire MG, Bressler SB, Fine SL, Alexander J et al. Occult choroidal neovascularization in age-related macular degeneration. Arch Ophthalmol 1997; 115: 345–350.
Thompson JT . Retinal pigment epithelial tear after transpupillary thermotherapy for choroidal neovascularization. Am J Ophthalmol 2001; 131: 662–664.
Benner JD, Ahuja RM, Butler JW . Macular infarction after transpupillary thermotherapy for subfoveal choroidal neovascularization in age-related macular degeneration. Am J Ophthalmol 2002; 134: 765–768.
Acknowledgements
We have no financial or proprietary interest in any of the products mentioned.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper was presented in part at the Club Jules Gonin Meeting, Athens 2004
Rights and permissions
About this article
Cite this article
Hogan, A., Kilmartin, D. Low power vs standard power transpupillary thermotherapy in patients with age-related macular degeneration and subfoveal choroidal neovascularization ineligible for photodynamic therapy. Eye 20, 649–654 (2006). https://doi.org/10.1038/sj.eye.6702028
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702028