Abstract
Aims To evaluate the involvement of multipotential stem and progenitor cells in the pathogenesis of pterygium.
Methods Paraffin-embedded and snap-frozen primary pterygium (n=10) were serially sectioned and analysed immunohistochemically to determine the expression level of AC133 (marker for the primitive haematopoietic progenitors), CD34 (marker for the haematopoietic progenitor cells and endothelium), c-Kit (marker for haematopoietic and stromal progenitor cells), and STRO-1 (a differentiation antigen present on bone marrow fibroblast cells and on various nonhaematopoietic progenitor cells).
Results In all the primary pterygium, immunoreactivity of AC133 and STRO-1 was found in some of the epithelial and stromal cells, CD34 was observed in the vascular endothelium, and some scattered ovoidal cells were found in the subepithelial connective tissue. C-Kit was expressed mainly in the basal epithelium of the head portions, and some spindle-shaped stromal cells. There is no immunoreactivity of AC133, c-Kit, and STRO-1 in normal conjunctiva, whereas CD34 was mildly stained with vessel wall.
Conclusion Multipotential stem and progenitor cells may be involved in the pathogenesis of pterygium through its differentiation into fibroblasts and vascular endothelial cells.
Similar content being viewed by others
Introduction
Pterygium is a chronic condition characterized by the encroachment of altered ocular surface tissue into the normal cornea. 1 Pterygium tissue consists of a superficial conjunctival epithelial layer and an underlying fibrovascular component, with features indicative of both degenerative process and disordered growth.2 The pathogenesis of the simple pterygium today remains an ophthalmic enigma. Although extensive literature exists on pterygium, controversy still surrounds the cell origin and the initial trigger in the development of this lesion; the relative roles of the pterygium fibroblast, epithelial cells, and limbal basal epithelial stem cell in the formation of this disease; and the mechanisms of pterygium's aggressive recurrence behaviour after surgical excision.
Despite the many classic and advanced theories of pterygium pathogenesis that have been reported, there is no consensus regarding the exact pathophysiological mechanisms. The multistep development of the tumour-like pterygium involves a tissue-remodelling process that includes increased expression of cytokines and overproliferation and differentiation of some cells. We hypothesize that certain environmental stimuli known to be associated with pterygium may stimulate bone marrow multipotential stem and progenitor cells and limbal stem cells to gain the unexpected potential ability to differentiate into cell types that are phenotypically unrelated to the origin, such as transformed fibroblast and altered epithelial cells, and that are capable of expressing various cytokines and stem cell-related factors associated with cell proliferation, angiogenesis, and tissue remodelling. These traits may be important for the formation of pterygium and its recurrence after surgical removal. The human haematopoietic progenitor cell antigen CD34 is known to be synthesized and expressed by certain cells of haematopoietic lineage.3, 4 Staining in tissue for CD34 is usually associated with vascular sprouting during vascularization.5 AC133 is another newly discovered early marker for primitive haematopoietic progenitors. CD34 cells that express AC133 are truly an immature population of haematopoietic stem and progenitor cells.6, 7 c-Kit is expressed only by activated bone marrow stromal cells.8 STRO-1 has recently been shown to mark multipotential bone marrow stromal cells.9 The purpose of this study was to advance the understanding of the cell origin of pterygium and the underlying mechanisms of recurrence, and to determine whether bone marrow-derived multipotential stem and progenitor cells are involved in the pathogenesis of pterygium.
Materials and methods
Specimen collection
All procedures followed the tenets of the Helsinki declaration, and written informed consent was obtained from all patients in the study. Specimens of pterygium were obtained after surgical removal (n=10). Specimens of normal human conjunctiva were obtained from healthy donors who did not have signs or symptoms of an ocular surface disorder or of dry eye. All tissue specimens were paraffin-embedded and snap-frozen. Serial cross-sections of pterygium were made along the longitudinal axis.
Immunohistochemical Analysis
Sectioned (2–4 μm) specimens were processed immunohistochemically, as previously described.10 Briefly, paraffin sections were deparafinized in xylene, rehydrated through decreasing graded ethanol, and quenched for endogenous peroxidase. Cryostat sections were placed on gelatinized slides, and then fixed in cold actone and rinsed in Tris-buffered saline. Nonspecific background was eliminated by incubating the tissue section with non-immuno serum (Zymed Laboratories, South San Francisco, CA, USA Histostain-plus Kits, Reagent A). Sections were then incubated with monoclonal mouse anti-human AC133 antibody (Clone AC133 epitope1, Miltenyi Biotech, Bergisch Gladbach, Germany), monoclonal mouse anti-human STRO-1 (DSHB, IA, USA), and polyclonal goat anti-human IgG primary antibodies: CD34 (N-19, Santa Cruz Biotechnology, Santa Cruz, CA, USA), c-Kit (C-14, Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C, then extensively washed in 0.05 M Tris-buffered saline (pH 7.6) before the addition of a biotinylated secondary antibody (Reagent B). Sections were again washed, incubated for 1 h with peroxidase-conjugated streptavidin (Reagent C), and the presence of peroxidase was revealed by adding substrate-chromogen (3-amino-9-ethycar bazole) solution (Reagent D). Sections were counterstained with haematoxylin.
Each section was photographed, and the entire tissue area was examined. The numbers of AC133, CD34, c-Kit, and STRO-1 positive cells were counted from three different regions of each sample under × 400 magnification. Statistical analyses were performed with SPSS version 11.5 for windows (SPSS Inc, Chicago, IL, USA).
Results
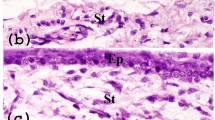
The blood vessels in the substantia propria displayed mild-to-moderate immunostaining for CD34 in the normal conjunctiva, whereas the epithelium of normal conjunctiva showed negative reaction for CD34. No AC133-, c-Kit-, or STRO-1-positive staining cells were observed in any part of the normal conjunctiva. In all the primary pterygium, immunoreactivity of AC133 was found in some of the round and ovoidal epithelial and stromal cells (Figure 1a). STRO-1 was expressed by many pterygium epithelial cells and stroma cells. Some of them exhibited a typical spindle-shape (Figure 1b). CD34 was observed in the endothelial cells of vascular cells and some oblong, ovoidal cells in subepithelial connective tissue. No staining was observed in the epithelium (Figure 1c). Staining indicative of the expression of c-Kit was found in the epithelium, especially along the base of the epithelium of the head portions of all the primary pterygium, and in some spindle-shaped or small and ovoidal-shaped cells in the subepithelial stromal tissue (Figure 1d).
The intensity of the AC133, CD34, c-Kit, and STRO-1 positive staining was variable among the 10 primary pterygium specimens. One of the 10 primary pterygium showed stronger reactivity for AC133, CD34, c-Kit, and STRO-1 than the other specimens. The staining pattern was similar to that of recurrent pterygium (data not shown). There were more STRO-1-positive cells (21.5±7.2) than CD34 (16.5±4.9) (t=3.151, P<0.05), than AC133 – (11.0±3.8) (t=7.056, P<0.001) and c-Kit – (15.9±5.0) (t=3.471, P<0.05) positive cells.
Discussion
The progressive nature of primary pterygium and its tendency to recur after surgical removal led us to further investigate the pathogenesis of this proliferative growth disorder, especially with regard to what kinds of cells initiate the formation of pterygium and are involved in its recurrence.
This present study demonstrated that AC133, CD34, c-Kit, and STRO-1 were expressed by primary pterygium cells. Leucocyte antigen CD34 is the common surface marker for the haematopoietic progenitor cells and endothelium,11 and it appears early in development as an endothelial cell surface receptor.12 The c-Kit proto-oncogene has been identified as a member of the receptor-tyrosine kinase family. When the c-Kit receptor binds with human stem cell factor (SCF), also termed c-Kit ligand, which is produced by mesenchymal cells, it will augment the proliferation of primitive bone marrow progenitor cells and differentiated mast cells.13, 14, 15 The fibroblast-like stem cells express the c-Kit, suggestive of mesenchymal differentiation. STRO-1 is a monoclonal antibody that has been shown to recognize all the clonogenic mesenchymal stem cells in human bone marrow. STRO-1 antigen is a differentiation antigen that is present on human bone marrow fibroblast-like cells and on various nonhaematopoietic progenitor cells.16, 17 In independent studies, a novel marker of human haematopoietic stem and progenitor cells was identified, characterized, and referred to as AC133 antigen.6, 7 The majority of cells reactive to the AC133 antibodies were found to co-express the human stem and progenitor marker CD34 and c-Kit.18, 19 Expression of AC133 is rapidly downregulated during cell differentiation. The AC133-positive cell population not only has haematopoietic potential, but also has the ability to differentiate into vascular endothelial cells.20 AC133 was expressed by pterygium epithelial and stromal cells. CD34-positive cells were not restricted to vascular endothelial cells, but also appeared in the subepithelial connective tissue. The expression pattern of c-Kit in primary pterygium was similar to that found by Nakagami,21 and it was expressed in pterygium epithelial cells and subepithelial stroma cells. The expression of progenitor cell's antigen on pterygium tissue is a sign of involvement of bone marrow-originated cells. We thus speculate that bone marrow-derived endothelial and stromal stem and progenitor cells are the likely candidates involved in pathogenesis of pterygium. The number of involved stem cells may be correlated with the risk of recurrence.
Based on the data presented in this investigation, we propose a theory for the pathogenesis of pterygium. Environmental factors, such as albedo UV light, heat, dryness, and wind, that have been regarded as important factors in the development of pterygium22, 23, 24, 25 cause limbal basal epithelial cells to be damaged,26 and trigger the secretion of proinflammatory mediators such as: TGF-β, tumour necrosis factor α (TNF-α), interleukin-1β, 6,8 (IL-1β, 6,8), and granulocyte colony-stimulating factor (GM-CSF).27, 28 These cytokines, on one hand, affect the production of matrix metalloproteinases (MMPs) by altered limbal basal cells and certain types of fibroblasts, to favour the degradation of extracellular matrix (ECM),29 thus enhancing the progressive corneal invasion of pterygium.30 On the other hand, these mediators and the damage to the corneal limbal area act as signals to bone marrow, stimulating the multistep repair and regenerative reaction by bone marrow multipotential stem and progenitor cells to heal corneal local damage. After they are stimulated, multipotential bone marrow stem and progenitor cells migrate through peripheral blood to the corneal limbal area. Under this special ocular microenvironment, these stem cells can be fully activated by expressing AC133, CD34, c-Kit, and STRO-1 receptor, and they produce certain cytokines and growth factors such as IL-6, IL-11, SCF, and GM-CSF.31 These factors may have both effect on themselves and limbal basal stem cells, resulting in differentiation into transformed pterygium fibroblast, epithelial cells, and endothelial cells.
Our hypothesis was consistent with the finding reported by Dushku that migration of altered limbal stem cells contributes to the pathogenesis of pterygium, and the vascular fibroplasias seen in pterygium is a response to a signal from the altered cells.32, 33 A recent study suggested that fibroblasts isolated from pterygium tissue exhibit a transformed phenotype.34 Our findings have relevance to explaining a new idea. The pivotal concept is that the infiltration of bone marrow AC133-, CD34-, c-Kit-, and STRO-1-positive stem cells may play an important role in the increase of transformed fibroblasts and new vascular formation of pterygium. Our data may help to explain why the subepithelial pterygium fibrovascular tissue, which is comprised of fibroblasts and capillary blood vessel cells, showed high proliferative ability.35 More STRO-1-positive cells than AC133, CD34, and c-Kit cells in our findings also showed the evidence that fibroblasts are the main component of the pterygium tissue.
Overexpression of AC133, CD34, c-Kit, and STRO-1 was detected in one of our 10 primary pterygium. Since, in certain abnormal ocular environments, infiltrated stem cells are frequently associated with differentiation and overproliferation, it is not unreasonable to postulate that the involvement of an increased number of stem cells may present an increased risk of recurrence of pterygium. If the pterygium is not completely removed by surgical excision, the stimulative condition will over time lead them to overdifferentiation and overproliferation of stem cells, thus contributing to more progressive and extensive fibrovascular formation in recurrent pterygium.
We demonstrate, for the first time, that the bone marrow-derived multipotential stem and progenitor cells are involved in the pathogenesis of pterygium, and are perhaps the important cells for both origin and recurrence of pterygium. In light of our findings, a pterygium grading system based on the infiltration of stem cells can be proposed to better predict the risk of recurrence clinically. The implication of our finding lies in devising a new possible strategy of pterygium treatment. A more focused approach to block stem cell activity and inhibit their overproliferative and overdifferentiative ability needs to be further studied.
References
Coroneo MT, Di Girolamo N, Wakefield D . The pathogenesis of pterygia. Curr Opin Ophthalmol 1999; 10: 282–288.
Kwok LS, Coroneo MT . A model for pterygium formation. Cornea 1994; 13: 219–224.
Boyd AW . Human leukocyte antigens: an update on structure, function and nomenclature. Pathology 1987; 19: 329–337.
Watt SM, Karhi K, Gatter K, Furley AJ, Katz FE, Healy LE et al. Distribution and epitope analysis of the cell membrane glycoprotein (HPCA-1) associated with human hemopoietic progenitor cells. Leukemia 1987; 1: 417–426.
Schlingemann RO, Rietveld FJ, de Waal RM, Bradley NJ, Skene AI, Davies AJ et al. Leukocyte antigen CD34 is expressed by a subset of cultured endothelial cells and on endothelial abluminal microprocesses in the tumor stroma. Lab Invest 1990; 62: 690–696.
Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT et al. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood 1997; 90: 5013–5021.
Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood 1997; 90: 5002–5012.
Huss R, Moosmann S . The co-expression of CD117 (c-kit) and osteocalcin in activated bone marrow stem cells in different diseases. Br J Haematol 2002; 118: 305–312.
Huss R, Moosmann S . The co-expression of CD117(c-kit) and osteocalcin in activated bone marrow stem cells in different diseases. Br J Haematol 2002; 118: 305–312.
Di Girolamo N, McCluskey PJ, Lloyd A, Coroneo MT, Wakefield D . Expression of MMPs and TIMPs in human pterygia and cultured pterygium epithelial cells. Invest Ophthalmol Vis Sci 2000; 41: 671–679.
Peault BM, Thiery JP, LeDouarin NM . Surface marker for hemopoietic and endothelial cell lineages that is defined by monoclonal antibody. Proc Natl Acad Sci USA 1983; 80: 2976–2980.
Garlanda C, Dejana E . Heterogeneity of endothelial cells: specific markers. Arterioscler Thromb Vasc Biol 1997; 17: 1193–1202.
Zsebo KM, Williams DA, Geissler EN, Broudy VC, Martin FH, Atkins HL et al. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell 1990; 63: 213–224.
Huang E, Nocka K, Beier DR, Chu TY, Buck J, Lahm HW et al. The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell 1990; 63: 225–233.
Anderson DM, Lyman SD, Baird A, Wignall JM, Eisenman J, Rauch C et al. Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane bound and soluble forms. Cell 1990; 63: 235–243.
Gronthos S, Graves SE, Ohta S, Simmons PJ . The STRO-1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood 1994; 84: 4164–4173.
Simmons PJ, Torok-Storb BSB . Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood 1991; 78: 55–62.
Majka M, Ratajczak J, Machalinski B, Carter A, Pizzini D, Wasik MA et al. Expression, regulation and function of AC133, a putative cell surface marker of primitive human hematopoietic cells. Folia Histochem Cytobiol 2000; 38: 53–63.
Wuchter C . Impact of CD133(AC133) and CD90 expression analysis for acute leukemia immunophenotyping. Haematologica 2001; 86: 154–161.
Schmeisser A, Strasser RH . Phenotypic overlap between hematopoietic cells with suggested angioblastic potential vascular endothelial cells. J Hematother Stem Cell Res 2002; 11: 69–79.
Nakagami T, Murakami A, Okisaka S, Ebihara N . Mast cells in pterygium: number and phenotype. Jpn J Ophthalmol 1999; 43: 75–79.
Wong WE . A hypothesis on the pathogenesis of pterygia. Am Ophthalmol 1978; 10: 303–308.
Coroneo MT . Pterygia as an early indicator of ultraviolet insolation: a hypothesis. Br J Ophthalmol 1993; 77: 734–739.
Cameron ME . Geographic distribution of pterygia. Trans Ophthalmol Soc Aust 1962; 22: 67–81.
Coroneo MT, Muller-Stolzenburg NW, Ho A . Peripheral light focusing by the anterior eye and the ophthalmohelioses. Ophthalmic Surg 1991; 22: 705–711.
Dushku N, Reid TW . P53 expression in altered limbal basal cells of pingueculae, pterygia and limbal tumors. Curr Eye Res 1997; 16: 1179–1192.
Gamache DA, Dimitrijevich SD, Weimer LK, Lang LS, Spellman JM, Graff G et al. Secretion of proinflammatory cytokines by human conjunctival epithelial cells. Ocular Immunol Inflam 1997; 5: 117–128.
Kennedy M, Kim KH, Brown J, Kennedy M, Kim KH, Harten B et al. Ultraviolet irradiation induces the production of multiple cytokines by human corneal cells. Invest Ophthalmol Vis Sci 1997; 38: 2483–2491.
Girolamo ND, Wakefield D, Coroneo MT . Differential expression of matrix metalloproteinases and their tissue inhibitors at the advancing pterygium head. Invest Ophthalmol Vis Sci. 2000; 41: 4142–4149.
Solomon A, Li DQ, Lee SB, Tseng SCG . Regulation of collagenase, stromelysin, and urokinase-type plasminogen activator in primary pterygium body fibroblasts by inflammatory cytokines. Invest Ophthalmol Vis Sci 2000; 41: 2154–2163.
Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL . Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res 2000; 9: 841–848.
Dushku N, John MK, Schultz GS, Reid TW . Pterygia pathogenesis: corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch Ophthalmol 2001; 119: 695–706.
Dushku N, Reid TW . Immunohistochemical evidence that human pterygia originate from an invasion of vimentin-expressing altered limbal epithelial basal cells. Curr Eye Res 1994; 13: 473–481.
Kria L, Ohira A, Amemiya T . Immunohistochemical localization of basic fibroblast growth factor, platelet derived growth factor, transforming growth factor-β and tumor necrosis factor-α in the pterygium. Acta Histochem 1996; 98: 195–201.
Tan DTH, Liu YP, Sun L . Flow cytometry measurements of DNA content in primary and recurrent pterygium. Invest Ophthalmol Vis Sci 2000; 41: 1684–1686.
Acknowledgements
This research was supported by research funds (code: stem cell 13) from Stem Cell Research Center of the 21C frontier research program funded by the Ministry of Science & Technology, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
The paper was presented in 2003 ARVO (1331/B227).
Rights and permissions
About this article
Cite this article
Ye, J., Song, Y., Kang, S. et al. Involvement of bone marrow-derived stem and progenitor cells in the pathogenesis of pterygium. Eye 18, 839–843 (2004). https://doi.org/10.1038/sj.eye.6701346
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6701346
Keywords
This article is cited by
-
Does systemic inflammation play a role in patients with pterygium?
International Ophthalmology (2020)
-
A new paradigm for stem cell therapy: Substance-P as a stem cell-stimulating agent
Archives of Pharmacal Research (2011)
-
A new role of substance P as an injury-inducible messenger for mobilization of CD29+ stromal-like cells
Nature Medicine (2009)
-
Temporary amniotic membrane patch for the treatment of primary pterygium: mechanisms of reducing the recurrence rate
Graefe's Archive for Clinical and Experimental Ophthalmology (2006)