Summary
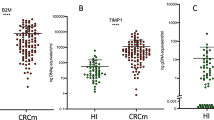
A kinetic enzyme-linked immunosorbent assay (ELISA) for plasma tissue inhibitor of metalloproteinase (TIMP)-1 was developed in order to examine the potential diagnostic and prognostic value of TIMP-1 measurements in cancer patients. The ELISA enabled specific detection of total TIMP-1 in EDTA, citrate and heparin plasma. The assay was rigorously tested and requirements of sensitivity, specificity, stability and good recovery were fulfilled. TIMP-1 levels measured in citrate plasma (mean 69.2 ± 13.1 μg l–1) correlated with TIMP-1 measured in EDTA plasma (mean 73.5 ± 14.2 μg l–1) from the same individuals in a set of 100 healthy blood donors (Spearman’s rho = 0.62, P < 0.0001). The mean level of TIMP-1 in EDTA plasma from 143 patients with Dukes’ stage D colorectal cancer was 240 ± 145 μg l–1 and a Mann–Whitney test demonstrated a highly significant difference between TIMP-1 levels in healthy blood donors and colorectal cancer patients (P < 0.0001). Similar findings were obtained for 19 patients with advanced breast cancer (mean 292 ± 331 μg l–1). The results show that TIMP-1 is readily measured in plasma samples by ELISA and that increased levels of TIMP-1 are found in patients with advanced cancer. It is proposed that plasma measurements of TIMP-1 may have value in the management of cancer patients.
Similar content being viewed by others
Article PDF
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Baker, T., Tickle, S., Wasan, H., Docherty, A., Isenberg, D. & Waxman, J. (1994). Serum metalloproteinases and their inhibitors: markers for malignant potential. Br J Cancer 70: 506–512.
Birkedal-Hansen, H., Moore, W. G., Bodden, M. K., Windsor, L. J., Birkedal-Hansen, B., DeCarlo, A. & Engler, J. A. (1993). Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 4: 197–250.
Clark, I. M., Powell, L. K., Wright, J. K. & Cawston, the (1991). Polyclonal and monoclonal antibodies against human tissue inhibitor of metalloproteinases (TIMP) and the design of an enzyme-linked immunosorbent assay to measure TIMP. Matrix 11: 76–85.
Cooksley, S., Hipkiss, J. B., Tickle, S. P., Holmes, I. E., Docherty, A. J., Murphy, G. & Lawson, A. D. (1990). Immunoassays for the detection of human collagenase, stromelysin, tissue inhibitor of metalloproteinases (TIMP) and enzyme-inhibitor complexes. Matrix 10: 285–291.
Cooper, T. W., Eisen, A. Z., Stricklin, G. P. & Welgus, H. G. (1985). Platelet-derived collagenase inhibitor: characterization and subcellular localization. Proc Natl Acad Sci USA 82: 2779–2783.
De Nictolis, M., Garbisa, S., Lucarini, G., Goteri, G., Masiero, L., Ciavattini, A., Garzetti, G. G., Stetler-Stevenson, W. G., Fabris, G., Biagini, G. & Prat, J. (1996). 72-kilodalton type IV collagenase, type IV collagen, and Ki 67 antigen in serous tumors or the ovary: a clinicopathologic, immunohistochemical, and serological study. Int J Gynecol Pathol 15: 102–109.
DeClerck, Y. A., Perez, N., Shimada, H., Boone, T. C., Langley, K. E. & Taylor, S. M. (1992). Inhibition of invasion and metastasis in cells transfected with an inhibitor of metalloproteinases. Cancer Res 52: 701–708.
Deng, G., Curriden, S. A., Wang, S., Rosenberg, S. & Loskutoff, D. J. (1996). Is plasminogen activator inhibitor-1 the molecular switch that governs urokinase receptor-mediated cell adhesion and release? J Cell Biol 134: 1563–1571.
Fong, K. M. (1996). TIMP1 and adverse prognosis in non-small cell lung cancer. Clin Cancer Res 2: 1369–1372.
Frandsen, T. L., Stephens, R. W., Pedersen, A. N., Engelholm, L. H., Holst-Hansen, C. & Brünner, N. (1998). Plasminogen activator inhibitor type 1 (PAI-1) in cancer: a potential new target for antiinvasive and antimetastatic therapy. Drugs of the Future 23: 873–883.
Fujimoto, N., Zhang, J., Iwata, K., Shinya, T., Okada, Y. & Hayakawa, T. (1993). A one-step sandwich enzyme immunoassay for tissue inhibitor of metalloproteinases-2 using monoclonal antibodies. Clin Chim Acta 220: 31–45.
Fung, K., Nowak, L., Lein, M., Henke, W., Schnorr, D. & Loening, S. A. (1996). Role of specimen collection in preanalytical variation of metalloproteinases and their inhibitors in blood. Clin Chem 42: 2043–2045.
Gohji, K., Fujimoto, N., Fujii, A., Komiyama, T., Okawa, J. & Nakajima, M. (1996). Prognostic significance of circulating matrix metalloproteinase-2 to tissue inhibitor of metalloproteinases-2 ratio in recurrence of urothelial cancer after complete resection. Cancer Res 56: 3196–3198.
Goldberg, G. I., Strongin, A., Collier, I. E., Genrich, L. T. & Marmer, B. L. (1992). Interaction of 92-kDa type IV collagenase with the tissue inhibitor of metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the proenzyme with stromelysin. J Biol Chem 267: 4583–4591.
Hayakawa, T., Yamashita, K., Tanzawa, K., Uchijima, E. & Iwata, K. (1992). Growth-promoting activity of tissue inhibitor of metalloproteinases-1 (TIMP-1) for a wide range of cells. A possible new growth factor in serum. FEBS Lett 298: 29–32.
Hembry, R. M., Murphy, G. & Reynolds, J. J. (1985). Immunolocalization of tissue inhibitor of metalloproteinases (TIMP) in human cells. Characterization and use of a specific antiserum. J Cell Sci 73: 105–119.
Jung, K., Nowak, L., Lein, M., Henke, W., Schnorr, D. & Loening, S. A. (1996). What kind of specimen should be selected for determining tissue inhibitor of metalloproteinase-1 (TIMP-1) in blood? Clin Chim Acta 254: 97–100.
Kawano, N., Osawa, H., Ito, T., Nagashima, Y., Hirahara, F., Inayama, Y., Nakatani, Y., Kimura, S., Kitajima, H., Koshikawa, N., Miyazaki, K. & Kitamura, H. (1997). Expression of gelatinase A, tissue inhibitor of metalloproteinases-2, matrilysin, and trypsin(ogen) in lung neoplasms: an immunohistochemical study. Hum Pathol 28: 613–622.
Khokha, R. & Waterhouse, P. (1993). The role of tissue inhibitor of metalloproteinase-1 in specific aspects of cancer progression and reproduction. J Neurooncol 18: 123–127.
Khokha, R., Zimmer, M. J., Graham, C. H., Lala, P. K. & Waterhouse, P. (1992a). Suppression of invasion by inducible expression of tissue inhibitor of metalloproteinase-1 (TIMP-1) in B16-F10 melanoma cells. J Natl Cancer Inst 84: 1017–1022.
Khokha, R., Zimmer, M. J., Wilson, S. M. & Chambers, A. F. (1992b). Up-regulation of TIMP-1 expression in B16-F10 melanoma cells suppresses their metastatic ability in chick embryo. Clin Exp Metastasis 10: 365–370.
Kleiner, Jr D. E., Tuuttila, A., Tryggvason, K. & Stetler-Stevenson, W. G. (1993). Stability analysis of latent and active 72-kDa type IV collagenase: the role of tissue inhibitor of metalloproteinases-2 (TIMP-2). Biochemistry 32: 1583–1592.
Kodama, S., Yamashita, K., Kishi, J., Iwata, K. & Hayakawa, T. (1989). A sandwich enzyme immunoassay for collagenase inhibitor using monoclonal antibodies. Matrix 9: 1–6.
Liotta, L. A., Steeg, P. S. & Stetler-Stevenson, W. G. (1991). Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 64: 327–336.
MacDougall, J. R. & Matrisian, L. M. (1995). Contributions of tumor and stromal matrix metalloproteinases to tumor progression, invasion and metastasis. Cancer Metastasis Rev 14: 351–362.
Matrisian, L. M. (1992). The matrix-degrading metalloproteinases. Bioessays 14: 455–463.
Mimori, K., Mori, M., Shiraishi, T., Fujie, T., Baba, K., Haraguchi, M., Abe, R., Ueo, H. & Akiyoshi, T. (1997). Clinical significance of tissue inhibitor of metalloproteinase expression in gastric carcinoma. Br J Cancer 76: 531–536.
Moll, U. M., Youngleib, G. L., Rosinski, K. B. & Quigley, J. P. (1990). Tumor promoter-stimulated Mr 92,000 gelatinase secreted by normal and malignant human cells: isolation and characterization of the enzyme from HT1080 tumor cells. Cancer Res 50: 6162–6170.
Moutsiakis, D., Mancuso, P., Krutzsch, H., Stetler-Stevenson, W. G. & Zucker, S. (1992). Characterization of metalloproteinases and tissue inhibitors of metalloproteinases in human plasma. Connect Tissue Res 28: 213–230.
Murphy, G., Houbrechts, A., Cockett, M. I., Williamson, R. A., O’Shea, M. & Docherty, A. J. (1991). The N-terminal domain of tissue inhibitor of metalloproteinases retains metalloproteinase inhibitory activity. Biochemistry 30: 8097–8102.
Nuovo, G. J., MacConnell, P. B., Simsir, A., Valea, F. & French, D. L. (1995). Correlation of the in situ detection of polymerase chain reaction-amplified metalloproteinase complementary DNAs and their inhibitors with prognosis in cervical carcinoma. Cancer Res 55: 267–275.
Stetler-Stevenson, W. G., Krutzsch, H. C. & Liotta, L. A. (1989). Tissue inhibitor of metalloproteinase (TIMP-2). A new member of the metalloproteinase inhibitor family. J Biol Chem 264: 17374–17378.
Stetler-Stevenson, W. G., Liotta, L. A. & Kleiner, Jr D. E. (1993). Extracellular matrix 6: role of matrix metalloproteinases in tumor invasion and metastasis. FASEB J 7: 1434–1441.
Stetler-Stevenson, W. G., Hewitt, R. & Corcoran, M. (1996). Matrix metalloproteinases and tumor invasion: from correlation and causality to the clinic. Semin Cancer Biol 7: 147–154.
Thorgeirsson, U. P., Lindsay, C. K., Cottam, D. W. & Gomez, D. E. (1993). Tumor invasion, proteolysis, and angiogenesis. J Neurooncol 18: 89–103.
Welgus, H. G., Jeffrey, J. J., Eisen, A. Z., Roswit, W. T. & Stricklin, G. P. (1985). Human skin fibroblast collagenase: interaction with substrate and inhibitor. Coll Relat Res 5: 167–179.
Wilhelm, S. M., Collier, I. E., Marmer, B. L., Eisen, A. Z., Grant, G. A. & Goldberg, G. I. (1989). SV40-transformed human lung fibroblasts secrete a 92-kDa type IV collagenase which is identical to that secreted by normal human macrophages. J Biol Chem 264: 17213–17221.
Yoshiji, H., Gomez, D. E. & Thorgeirsson, U. P. (1996). Enhanced RNA expression of tissue inhibitor of metalloproteinases-1 (TIMP-1) in human breast cancer. Int J Cancer 69: 131–134.
Zucker, S., Lysik, R. M., DiMassimo, B. I., Zarrabi, H. M., Moll, U. M., Grimson, R., Tickle, S. P. & Docherty, A. J. (1995). Plasma assay of gelatinase B: tissue inhibitor of metalloproteinase complexes in cancer. Cancer 76: 700–708.
Author information
Authors and Affiliations
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Holten-Andersen, M., Murphy, G., Nielsen, H. et al. Quantitation of TIMP-1 in plasma of healthy blood donors and patients with advanced cancer. Br J Cancer 80, 495–503 (1999). https://doi.org/10.1038/sj.bjc.6690384
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6690384
Keywords
This article is cited by
-
Overexpression of TIMP-1 and Sensitivity to Topoisomerase Inhibitors in Glioblastoma Cell Lines
Pathology & Oncology Research (2019)
-
Comparative studies of TIMP-1 immunohistochemistry, TIMP-1 FISH analysis and plasma TIMP-1 in glioblastoma patients
Journal of Neuro-Oncology (2016)
-
Serum metalloproteinase-9 is related to COPD severity and symptoms - cross-sectional data from a population based cohort-study
Respiratory Research (2015)
-
TIMP-1 and CEA as biomarkers in third-line treatment with irinotecan and cetuximab for metastatic colorectal cancer
Tumor Biology (2015)
-
Detection of serological biomarkers by proximity extension assay for detection of colorectal neoplasias in symptomatic individuals
Journal of Translational Medicine (2013)