Abstract
Background:
Here, we explore the therapeutic potential of dasatinib, a small-molecule inhibitor that targets multiple cytosolic and membrane-bound tyrosine kinases, including members of the Src kinase family, EphA2, and focal adhesion kinase for the treatment of ovarian cancer.
Methods:
We examined the effects of dasatinib on proliferation, invasion, apoptosis, cell-cycle arrest, and kinase activity using a panel of 34 established human ovarian cancer cell lines. Molecular markers for response prediction were studied using gene expression profiling. Multiple drug effect/combination index (CI) isobologram analysis was used to study the interactions with chemotherapeutic drugs.
Results:
Concentration-dependent anti-proliferative effects of dasatinib were seen in all ovarian cancer cell lines tested, but varied significantly between individual cell lines with up to a 3 log-fold difference in the IC50 values (IC50 range: 0.001–11.3 μmol l−1). Dasatinib significantly inhibited invasion, and induced cell apoptosis, but less cell-cycle arrest. At a wide range of clinically achievable drug concentrations, additive and synergistic interactions were observed for dasatinib plus carboplatin (mean CI values, range: 0.73–1.11) or paclitaxel (mean CI values, range: 0.76–1.05). In this study, 24 out of 34 (71%) representative ovarian cancer cell lines were highly sensitive to dasatinib, compared with only 8 out of 39 (21%) representative breast cancer cell lines previously reported. Cell lines with high expression of Yes, Lyn, Eph2A, caveolin-1 and 2, moesin, annexin-1, and uPA were particularly sensitive to dasatinib.
Conclusions:
These data provide a clear biological rationale to test dasatinib as a single agent or in combination with chemotherapy in patients with ovarian cancer.
Similar content being viewed by others
Main
Ovarian cancer is a leading cause of cancer death among women in the United States and Western Europe and has the highest mortality rate of all gynaecologic cancers. Despite the fact that maximal cytoreductive surgery followed by combination chemotherapy increases 5-year survival rates, the overall survival gains have been modest with low overall cure rates (Aletti et al, 2007). Thus, novel systemic treatment approaches are urgently needed. Src is the prototypic member of a family of nine non-receptor tyrosine kinases (Src, Lyn, Fyn, Lck, Hck, Fgr, Blk, Yrk, and Yes) that has a key role in many cellular signalling pathways (reviewed in Thomas and Brugge, 1997). Src family kinase (SFK) proteins regulate four main cellular functions that ultimately control the behaviour of transformed cells: cell proliferation, adhesion, invasion, and motility (Summy and Gallick, 2003; Yeatman, 2004). Interactions with activated EGFR (Tice et al, 1999), HER2 (Muthuswamy et al, 1994), fibroblast growth factor receptor (Landgren et al, 1995), or hepatocyte growth factor (Mao et al, 1997) can result in Src activation, most likely by disrupting the intramolecular interactions that hold Src in a closed and inactive configuration (Thomas and Brugge, 1997). Importantly, aberrant expression and activation of the SFKs have been described in human ovarian cancer cell lines (Budde et al, 1994) and clinical samples (Wiener et al, 2003). A recent study used gene expression signatures that define the status of Src signalling pathways to predict the probability of Src pathway activation in 119 patients with ovarian cancer (Dressman et al, 2007). Approximately half of the tumours examined in this study showed Src pathway deregulation. Furthermore, those patients with a deregulated Src pathway also showed the worst prognosis.
Dasatinib is a small molecule that inhibits not only recombinant kinase domains of SFKs such as Src, Lck, and Yes, but also other tyrosine kinases such as Abl, c-Kit, and platelet-derived growth factor receptor-β (PDGFR-β) by 50% (IC50) at low nanomolar concentrations (Lombardo et al, 2004). Moreover, recent studies have also identified focal adhesion kinase (FAK; Bantscheff et al, 2007) and EphA2 (Huang et al, 2007) as direct targets of dasatinib. Focal adhesion kinase is a non-receptor tyrosine kinase involved in the regulation of cell adhesion, survival, and migration, which itself can be activated by Src (Fincham and Frame, 1998; Schaller, 2001). Importantly, preclinical studies indicate that FAK has a significant role in ovarian cancer cell migration and invasion (Sood et al, 2004). The Eph receptor tyrosine kinases and ephrin ligands have been studied extensively for their roles in developmental processes. However, in recent years, Eph receptors and ephrins have been found to be integral players in cancer formation and progression (Wykosky and Debinski, 2008). Eph2A, as well as other members of the Eph receptor tyrosine kinase family, have been associated with advanced ovarian cancer and poor clinical outcome (Thaker et al, 2004; Wu et al, 2006; Kumar et al, 2007; Alam et al, 2008). On the basis of these data, we hypothesise that dasatinib, which targets SFKs as well as FAK and Eph2A, may show significant preclinical activity in ovarian cancer cells supporting its further clinical evaluation. Thus far, dasatinib has proven to be clinically active in patients with imatinib-refractory chronic myelogenous leukaemia and Philadelphia chromosome-positive ALL (Talpaz et al, 2006). Moreover, dasatinib has also recently shown preclinical activity in prostate cancer cells (Nam et al, 2005), basal-like breast cancer cells (Finn et al, 2007), melanoma cells (Eustace et al, 2008), colon cancer cells (Serrels et al, 2006), and head and neck squamous cancer cells or non-small-cell lung cancer cells (Johnson et al, 2005; Song et al, 2006). Inhibitory effects of dasatinib on cell migration and invasion of epithelial tumour cells have been well documented in these studies, indicating that dasatinib may not only have anti-proliferative but also anti-invasive and anti-metastatic activity in epithelial tumours.
Our primary aim was to more comprehensively explore the growth-inhibitory effects of this multikinase inhibitor using a large panel of ovarian cancer cell lines, representing all histological subtypes of the disease. To more fully understand the activity, we also studied the effects of dasatinib on cell cycle invasion and apoptosis. Furthermore, we sought to validate previously reported, as well as identify novel response markers, all of which are known to be implicated in Src signalling. Finally, we used multiple drug effect/combination index (CI) isobologram analysis to study the efficacy of chemotherapeutic drugs plus dasatinib combinations tested against dasatinib-sensitive ovarian cancer cells. The current studies were intended to provide a rationale to test dasatinib as a single agent or in combination with chemotherapy in patients with ovarian cancer and to identify molecular markers that may help define subsets of ovarian cancer patients most likely to benefit from treatment with dasatinib.
Materials and methods
Cell lines, cell culture, and reagents
The cell lines CAOV3, ES2, OV90, TOV112D, TOV21G, SW626, and OVCAR3 were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). The cell lines A2780, OAW28, and OAW42 were obtained from the European Collection of Cell Cultures (Salisbury, UK). The cell lines COLO704, EFO21, and EFO27 were obtained from the German Tissue Repository DSMZ (Braunschweig, Germany). The cell lines MCAS, OVISE, OVKATE, OVMANA, OVSAHA, OVTOKO, and RMG1 were obtained from the Japanese Health Science Research Resources Bank (Osaka, Japan). The cell lines OVCAR5, OV167, OV177, OV207, DOV13, and HEYC2 were a kind gift from Dr V Shridhar (Mayo Clinic, Rochester, MN, USA). The cell line PE06 was a kind gift from Dr SP Langdon (Edinburgh Cancer Research Center, University of Edinburgh, Edinburgh, UK). The cell line HEY was a kind gift from Dr DT Curiel (University of Alabama at Birmingham, Birmingham, AL, USA). The cell lines SKOV3, OV2008, OVCA420, OVCA429, OVCA432, and OVCA433 were a kind gift from Dr B Karlan (Cedars Sinai, Los Angeles, CA, USA). The individuality of each cell line was checked by mitochondrial DNA sequencing. OV167, OV177, and OV207 cells were cultured in MEM medium (Fisher Scientific, Pittsburgh, PA, USA) plus 20% fetal bovine serum (FBS). OVCA420 and OVCA429 cells were cultured in MEM medium (Fisher Scientific) plus 10% FBS. EFO27 and EFO21 cells were cultured in RPMI 1640 medium (ATCC) plus 20% FBS and 1% MEM Non-Essential Amino Acid Solution (ATCC). COLO704, HEY, OV2008, OVISE, OVMANA, OVTOKO, OVKATE, and OVSAHO cells were cultured in RPMI 1640 medium plus 10% FBS. MCAS, DOV13, OVCA432, and OVCA433 cells were cultured in Eagle's minimal essential medium (ATCC) plus 15% FBS. RMG1 cells were cultured in Ham's F12 (ATCC) plus 10% FBS. SW626 cells were cultured in Leibovitz's L-15 medium (ATCC) plus 10% FBS. HEYC2 and PE06 cells were cultured in DMEM/F12 (ATCC) plus 10% FBS. OAW42 and OAW28 cells were cultured in DMEM (ATCC) plus 10%FBS. ES2 and SKOV3 were cultured in McCoy's 5A medium (ATCC) plus 10% FBS. OVCAR5, OVCAR3, and CAOV3 cells were cultured in DMEM medium plus 10% FBS and 4.5 g l−1 glucose. A2780 cells were cultured in RPMI 1640 medium plus 10% FBS and 0.25 U ml−1 insulin. OV90, TOV21G, and TOV112D cells were cultured in a 1 : 1 mixture of MCDB 105 medium (Cell Applications Inc., San Diego, CA, USA) and Medium 199 (Sigma-Aldrich, St Louis, MO, USA) plus 15% FBS. All cell line cultures were supplemented with 1% penicillin–streptomycin and 1% L-glutamine. Dasatinib was provided by Bristol-Myers Squibb (New York, NY, USA) and prepared as a 10 mmol l−1 concentrated stock solution in DMSO.
Proliferation assays
Cells were plated into 24-well tissue culture plates at a density of 2 × 105 to 5 × 105 and grown in cell-line-specific medium without or with increasing concentrations of dasatinib (ranging between 0.001 and 10 μmol l−1). Cells were counted on day 7 using an automated cell viability assay (Vi-CELL XR Cell Viability Analyzer, Beckman Coulter, Fullerton, CA, USA), which is a video imaging system using an automated trypan blue exclusion protocol. Both adherent and floating viable cells were counted for treatment and control wells. Growth inhibition (GI) was calculated as a percentage of untreated controls. The log of the fractional GI was then plotted against the log of the drug concentration and the IC50 values were interpolated from the resulting linear regression curve fit (CalcuSyn; Biosoft, Ferguson, MO, USA). Experiments were performed thrice in duplicate for each cell line.
Cell cycle analysis
Cells were plated in six-well tissue culture plates and treated with vehicle (0.1% DMSO) or 1 μmol l−1 dasatinib for 72 h. After collection, the cells were fixed in 70% ethanol, incubated in HCl/Triton X-100, and stained with propidium iodide. Samples were analysed by flow cytometry (Becton Dickinson, San Jose, CA, USA) according to the manufacturer's protocol.
Annexin V and propidium iodide flow cytometry
Cells were treated with 1 μmol l−1 dasatinib for 5 days. Apoptotic subpopulations were detected by labelling phosphatidylserine residues of the cell surface with annexin V-FITC and staining cells with propidium iodide. Detection was carried out on floating and adherent cell fractions by two-colour flow cytometry (Becton Dickinson) according to the manufacturer's protocol.
Invasion assay
Invasion assays were carried out as previously described (Glynn et al, 2004). Briefly, Matrigel (BD Biosciences, San Jose, CA, USA) was diluted to 1 mg ml−1 in serum-free medium. A volume of 100 ml of Matrigel was placed into each insert (Falcon) (8.0 mm pore size), which stood in wells of a 24-well plate (Costar, Lowell, MA, USA). The inserts and the plate were incubated for 5 h at 37°C for gelling. Cells were then suspended in serum-free culture media at a concentration of 106 ml−1. Subsequently, 100 ml of the cell suspension was added to each insert and 500 ml culture media containing 10% FBS was added to the bottom well. Cells were incubated at 37°C in culture media with or without 1 μmol l−1 dasatinib for 48 h. After this time period, the top side of the insert was wiped with a wet swab to remove the cells and the insert was stained with 0.25% crystal violet. Cells entering the Matrigel layer were viewed at × 200 magnification and counted in four random fields per sample. All assays were carried out in triplicate.
Western blot and immunoprecipitation
Cells were washed in PBS and lysed at 4°C in lysis buffer. Insoluble material was cleared by centrifugation at 10 000 g for 10 min. Protein was quantitated using BCA (Pierce, Rockford, IL, USA), resolved by SDS–PAGE, and transferred to nitrocellulose membranes (Invitrogen Life Technologies, Carlsbad, CA, USA). Phosphorylated Src was detected by an anti-pSrc antibody (Tyr416, Calbiochem, San Diego, CA, USA). Phosphorylated FAK was detected using an anti-pFAK antibody (Tyr576/577, Invitrogen Life Technologies). Tyrosine phosphorylation of Yes, Lyn, and Eph2A was analysed as follows. Immunoprecipitations were performed by allowing 250 μg protein lysate to incubate with 3 μg anti-Yes (C-10, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-Lyn (H-70, Santa Cruz Biotechnology), and anti-EphA2 antibodies (L-20, Santa Cruz Biotechnology) and protein A/G-agarose (Santa Cruz Biotechnology) at 4°C overnight with gentle agitation. The immunoprecipitates were washed thrice in lysis buffer and then denatured in Laemmli buffer before SDS–PAGE. Immunoblotting was performed using a monoclonal anti-phosphotyrosine antibody (Y20, Becton Dickinson Biosciences, San Jose, CA, USA). Detection was carried out using enhanced chemiluminescence (ECL; Amersham Biosciences UK, Buckinghamshire, England).
Multiple drug effect analysis
Aliquots of 3 × 103 to 5 × 103 OVCAR3, OVCAR5, RMG1, and SKOV3 cells were plated in 96-well microdilution plates. After cell adherence (24 h), experimental medium containing control medium, dasatinib, carboplatin or paclitaxel, or the combination (dasatinib plus carboplatin or paclitaxel) was added to appropriate wells in duplicate, and serial twofold dilutions were performed to span clinically relevant concentration ranges for the dose-effect analysis for dasatinib and chemotherapeutic agent or drug combination. Multiple drug effect analysis was carried out as previously described (Pegram et al, 2004). Combination index values were derived from variables of the median effect plots and statistical tests were applied (unpaired, two-tailed Student's t-test) to determine whether the mean CI values at multiple effect levels were significantly different from CI=1. In this analysis, synergy is defined as CI values significantly lower than 1.0, antagonism as CI values significantly higher than 1.0, and additivity as CI values equal to 1.0.
Gene expression profiling of ovarian cell lines
Agilent microarray analyses were developed for each cell line. Briefly, cells were grown to log phase and then RNA was extracted using the RNeasy Kit (Qiagen, Valencia, CA, USA). The purified RNA was eluted in 30–60 ll DEPC water and the quantity of RNA measured by spectral analysis using the Nanodrop Spectrophotometer. RNA quality was determined by separation of the RNA through capillary electrophoresis using the Agilent 2000 Bioanalyzer (Agilent, Santa Clara, CA, USA). Microarray hybridisations of 34 ovarian cell lines were performed using the Agilent Human 1A V2 array. Characterisation of individual ovarian cancer cell lines by comparison with an ovarian cancer cell line mixed reference pool was conducted on a single slide in which the mixed pool RNA was labelled with cyanine-3 and the individual cell lines with cyanine-5. The mixed reference pool consisted of equal amounts of cRNA from all ovarian cancer cell lines examined. Microarray slides were read using an Agilent Scanner and the Agilent Feature Extraction software version 7.5 was used to calculate gene expression values. The feature-extracted files were imported into the Rosetta Resolver system for gene expression data analysis (version 7.2; Rosetta Biosoftware, Seattle, WA, USA). The intensity ratios between the cell line sample and mixed reference were calculated for each sequence and computed according to the Agilent error model. A particular sequence was considered differentially expressed if the calculated P-value was ⩽0.01.
Statistical methods
The Resolver System ratio-splitting operation was used to generate intensity profiles from the set of ovarian cell line ratio scans and breast cell line ratio scans previously described (Finn et al, 2007). The ratio-splitting operation includes error modelling of the channels of each ratio scan, group normalisation, forward transformation of intensities, group detrending, inter-slide error correction, and finally inverse transformation of intensities. After ratio splitting, the ovarian cell lines and breast cell lines were clustered across a set of genes related to dasatinib sensitivity. The two-dimensional cluster analysis was carried out using an agglomerative hierarchical clustering algorithm based on the cosine correlation similarity metric. Before clustering, the intensity values were converted to Z-scores using the Resolver System data processing pipeline. The Z-score transformation converts the mean for each gene to zero and the standard deviation is set to 1. The cluster data were exported, and associations between the expression levels of biomarkers (Z-scores) and GI were analysed using Spearman's ρ-correlation, and differences between the subgroups were compared using Student's t-test. Cells with ⩾60% GI were classified as highly sensitive, cells with 40–59% GI as moderately sensitive, and cells with <40% GI as resistant. All statistical tests were two-sided.
Results
Activity of dasatinib in ovarian cancer cells
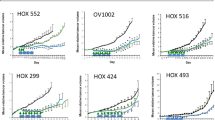
The effects of dasatinib on human ovarian cancer cells were evaluated using a panel of 34 established human ovarian cancer cell lines. These cells lines were selected to be representative of a range of ovarian cancer subtypes. Of these cell lines, 18 cell lines were obtained from patients with serous papillary ovarian cancer, 8 cell lines from patients with clear cell ovarian cancer, and 4 cell lines from patients with undifferentiated ovarian cancer. The remaining four cell lines were obtained from patients with endometrioid or mucinous ovarian cancer. Earlier reports indicate that dasatinib may inhibit cell-surface adhesion of epithelial tumour cells grown in culture (Nam et al, 2005; Serrels et al, 2006). To study the influence of dasatinib treatment on the detachment of viable cells grown in culture, we compared the fraction of floating cells between dasatinib-treated and control cells. Our results indicate that dasatinib can slightly increase the fraction of floating viable cells grown in culture (Figure 1). Therefore, both adherent and floating viable cells were analysed in the cell count experiments used for assessing GI. The effective dose range (IC10–IC80) was identified using a wide range of dasatinib concentrations (0.001–10 μmol l−1). Dasatinib inhibited the proliferation of all ovarian cancer cell lines investigated in a concentration-dependent manner; however, the IC50 values varied significantly between individual cell lines with up to a 3 log-fold difference in the IC50 values and ranged between 0.001 μmol l−1 in HEY ovarian cancer cells and 11.3 μmol l−1 in OVKATE ovarian cancer cells (Table 1 and Figure 2). When comparing the sensitivity with dasatinib between histological subtypes, none of the serous papillary, clear cells, or the endometrioid, mucinous, or undifferentiated ovarian cancer cells were preferentially sensitive to dasatinib (data not shown). We have previously reported on the growth inhibitory effect of dasatinib in a large representative panel of 39 breast cancer cell lines (Finn et al, 2007). When treated with dasatinib in vitro, 8 (21%) of them were highly sensitive (⩾60% GI), 10 of them were moderately sensitive (40–59% GI), and 21 were resistant (<40% GI) to dasatinib. The results of this study indicate that 24 out of the 34 (71%) ovarian cancer cell lines were highly sensitive (⩾60% GI), 6 of them were moderately sensitive (40–59% GI), and only 4 were resistant to dasatinib (<40% GI). Importantly, we used the identical cell proliferation assays for assessing GI in both the breast and the ovarian cancer cell line panels.
The influence of dasatinib treatment on the detachment of viable cells grown in culture was studied by comparing the fraction of floating cells between dasatinib-treated and control cells. Cells were treated with 0.5 μmol l−1 dasatinib. Cells were counted on day 3 by an automated cell viability assay using an automated trypan blue exclusion protocol. Both adherent and floating viable cells were counted for treatment and control wells. Our results indicate that dasatinib can slightly increase the fraction of floating viable cells grown in culture. Therefore, both adherent and floating viable cells were analysed in the cell count experiments used for assessing growth inhibition.
Growth inhibitory effects of dasatinib were studied across a panel of ovarian cancer cell lines. Cells were grown in cell line-specific media without or with increasing doses of dasatinib (ranging between 0.001 and 10 m mol l−1). Cells were trypsinised and counted after 7 days of treatment. The percentage of inhibition was calculated compared with untreated controls. The log of the fractional growth inhibition (GI) was plotted against the log of the drug concentration. The dose achieving 50% GI (IC50) was interpolated from the resulting linear regression curve fit. Cell lines are ordered from left to right from low to high IC50 values. Error bars indicate the s.e. of the mean value. Mean is derived from three replicate experiments.
Predictors of dasatinib response in vitro
A key aspect of therapies with targeted agents is the accurate selection of patients most likely to benefit from therapy. Earlier reports have shown that high expression of annexin-1, caveolin-1, caveolin-2, moesin, and uPA, as well as low expression of IGFBP2, was associated with the in vitro response to dasatinib in breast, lung, and prostate cancer cells (Finn et al, 2007; Huang et al, 2007; Wang et al, 2007). Here, we explored the association between the various biomarkers implicated in Src signalling pathways and the in vitro response to dasatinib in 73 human cancer cell lines, including the current 34 ovarian cancer cell lines and 39 breast cancer cell lines that have been published earlier (Finn et al, 2007). Restricting such a correlative analysis between the in vitro response and differential gene expression to only ovarian cancer cell lines would be of limited value as most ovarian cancer cell lines were highly sensitive and only four ovarian cancer cell lines showed resistance (defined as <40% GI) and only seven ovarian cancer cell lines showed an IC50 >1 μ M. Importantly, in vitro sensitivity towards dasatinib was assessed using the same methodology in the ovarian and breast cancer cell line panels, and cell lines were uniformly classified as highly sensitive (⩾60% GI), moderately sensitive (40–59% GI), and resistant to dasatinib (<40% GI). Of the known dasatinib targets, high expression of Yes, Lyn, and EphA2 but not of Src, FAK, Kit, or PDGFR-β was associated with in vitro sensitivity to dasatinib (Figure 3, Table 2). Furthermore, our findings corroborate earlier data, in that cell lines with high expression of annexin-1, caveolin-1, caveolin-2, moesin, and uPA but low expression of IGFBP2 were the most sensitive to dasatinib (Figure 3, Table 2). We also studied additional markers such as E-cadherin, P-cadherin, and N-cadherin that have an important role in cell adhesion, as well as β-catenin and δ-catenin/p120, both found in complexes with cadherin molecules. Our results indicate that cell lines with high expression of E-cadherin or P-cadherin, as well as δ-catenin/p120, were less sensitive to dasatinib when compared with those with lower expression of the respective markers (Figure 3, Table 2). We also studied the expression of receptor tyrosine kinases such as HER2 and MET or components of the VEGF/STAT3 signalling pathway, which can all signal through Src (Mukhopadhyay et al, 1995; Rahimi et al, 1998; Laird et al, 2003; Vadlamudi et al, 2003). Surprisingly, high expression of HER2, MET, or VEGF and STAT3 was not associated with in vitro sensitivity to dasatinib; in fact, high expression of HER2, VEGF, and STAT3 was rather correlated with in vitro resistance to dasatinib.
Microarray hybridisations of 34 ovarian cell lines were performed using the Agilent Human 1A V2 array. Characterisation of individual ovarian cancer cell lines by comparison with an ovarian cancer cell line mixed reference pool was conducted on a single slide in which the mixed pool RNA was labelled with cyanine-3 and the individual cell lines with cyanine-5. The mixed reference pool consisted of equal amounts of cRNA from all ovarian cancer cell lines examined. The Resolver System was used to generate intensity profiles from the set of ovarian cell line ratio scans and breast cell line ratio scans previously described (Finn et al, 2007). The red and green matrices represent the normalised expression patterns for each gene in Table 2 across the 34 ovarian and 39 breast cancer cell lines. Brightest red indicates the highest relative expression and brightest green indicates the lowest relative expression. The bottom 2 rows are matrices that represent the in vitro sensitivity to dasatinib (blue=highly sensitive, ⩾60% growth inhibition (GI); red=moderately sensitive, 40–59% GI; and green=resistant, <40% GI), and the cell line origin (yellow=ovarian cancer cell line; blue=breast cancer cell line).
Effects of dasatinib on ovarian cancer cell cycling, survival, and invasion
Earlier reports have suggested that dasatinib may have anti-proliferative activity by inducing cell cycle arrest and apoptosis in lung or head and neck cancer cells (Johnson et al, 2005; Song et al, 2006), malignant pleura mesothelioma (Tsao et al, 2007), or melanoma cells (Eustace et al, 2008). Here, we confirm and extend these findings to ovarian cancer cells. At a concentration of 1 μmol l−1, dasatinib treatment significantly increased the fraction of early and late apoptotic cells in the human ovarian cancer cell lines examined (Figure 4). In contrast, however, treatment with 1 μmol l−1 dasatinib over 72 h only lead to a modest increase in the fraction of cells in the G0/G1 cell cycle phase in only one out of the four examined human ovarian cancer cell lines when compared with the untreated controls (Figure 5). At a concentration of 1 μmol l−1, dasatinib treatment also significantly inhibited the ability of ovarian cancer cells to invade matrigel when compared with untreated controls in all of the four examined cell lines (Figure 6).
Detection of apoptotic subpopulations was achieved by labelling phosphatidylserine residues of the cell surface with annexin V-FITC and staining cells with propidium iodide. Cells were treated with 1 μmol l−1 dasatinib for 5 days. This staining allows differentiation between early (Q4) and late (Q2) apoptotic subpopulations.
Invasion was assessed using Matrigel invasion assays as described in Materials and methods. Cells were incubated at 37°C in culture media with or without 1 μmol l−1 dasatinib for 48 h. Cells invading the Matrigel layer were viewed at × 200 magnification and counted in four random fields per sample. All assays were performed in triplicate.
Effects of dasatinib on Src, Yes, Lyn, FAK, and Eph2A phosphorylation
We assessed the effect of dasatinib on the phosphorylation state of Src, Lyn, Yes, EphA2, and FAK using immunoblotting techniques. Exposure of EFO27, CAOV3, OVKATE, OVCAR3, A2780, COLO704, OV167, and OVCAR5 cells to 1 μmol l−1 dasatinib for 1 h resulted in a pronounced reduction of pSrc, pYes, pLyn, pEphA2, and pFAK (Figure 7).
Effect of dasatinib on the phosphorylation state of Src, Lyn, Yes, EphA2, and FAK using immunoblotting techniques. Exposure of EFO27, CAOV3, OVKATE, OVCAR3, A2780, COLO704, OV167, and OVCAR5 cells to 1 μmol l−1 dasatinib for 1 h resulted in a pronounced reduction of pSrc, pYes, pLyn, pEphA2, and pFAK.
Combination of dasatinib and chemotherapeutic agents
We next analysed the combination of dasatinib and chemotherapeutic agents used for the treatment of ovarian cancer. Multiple drug effect analysis was performed using four dasatinib-sensitive ovarian cancer cell lines to determine the nature of the interaction between dasatinib and carboplatin or paclitaxel (synergy, addition, or antagonism). The dasatinib concentrations used for these experiments ranged between 0.031 and 0.5 μmol l−1 for OVCAR5, 0.050 and 0.8 μmol l−1 for SKOV3 cells, 0.008 and 0.125 μmol l−1 for RMG1, and 0.016 and 0.250 μmol l−1 for CAOV3 cells and were below the reported peak plasma concentrations achievable in humans (Wang et al, 2008). The drug concentrations of carboplatin and paclitaxel used for these experiments were similarly below the reported peak plasma concentrations that have been previously published (Pegram et al, 2004). Synergistic and additive interactions were observed for dasatinib plus carboplatin (mean CI values ranged from 0.73 (95% CI: 0.56–0.90, P=0.002) in RMG1 cells to 1.11 (95% CI: 0.77–1.46, P=0.553) in OVCAR5 cells) and for dasatinib plus paclitaxel (0.76 (95% CI: 0.54–0.99, P=0.016) in SKOV3 cells to 1.05 (95% CI: 0.87–1.23, P=0.524) in OVCAR5 cells) (Figure 8).
Mean CI values for chemotherapeutic drug–dasatinib combinations in four different human ovarian cancer cell lines. Error bars indicate the 95% confidence interval of the mean value. Mean is derived from three replicates spanning clinically relevant concentration ranges sufficient to inhibit growth of control cells by 20–90%. Combination index values were derived from parameters of the median effect plots, and statistical tests were used to determine whether the CI values at multiple effect levels (IC20–IC90) were statistically significantly different from CI values equal to 1. Values that are statistically significantly <1 indicate synergistic interactions. Values that are statistically significantly >1 indicate antagonistic interactions. Values equal to (or not statistically significantly different from) 1 indicate additive interactions.
Discussion
Although the Src kinase has been linked with the development and progression of cancer for many years, there has only recently been a renewed interest in this onco-protein as a molecular target for cancer therapy. Src family kinases modulate signal transduction through multiple oncogenic pathways, including EGFR, HER2, PDGFR, FGFR, and VEGFR (Yeatman, 2004). In turn, a wide range of Src substrates have been identified that regulate cell growth, adhesion, invasion, and motility (Thomas and Brugge, 1997; Yeatman, 2004). Thus, it is anticipated that blocking Src kinase would be predicted to have a broad therapeutic benefit in patients with Src-dependent cancers. Dasatinib is currently being tested or entering clinical trials in various epithelial malignancies (http://ClinicalTrials.gov). However, despite the ubiquitous expression of SFKs, they may have varying roles in different epithelial cancers and, moreover, may be more important in specific disease subgroups compared with others. Here, we are able to show that approximately two-thirds of the ovarian cancer cell lines examined showed high in vitro sensitivity to dasatinib, which is in stark contrast to the fact that similar sensitivity was only seen in one-fifth of the breast cancer cell lines examined. Nevertheless, we still found a wide range of in vitro response to dasatinib with up to a threefold log difference in IC50 values between the most sensitive and the most resistant cell lines. We therefore sought to validate biomarkers that predict in vitro response to dasatinib. In doing so, we focused primarily on biomarkers that have been reported earlier or that are known to be implicated in Src signalling. We intended to identify a limited number of individual response markers that may be more useful for independent validation in future clinical studies. Our results confirm data from earlier studies that were obtained in breast, lung, and prostate cancer cell lines, in that high expression levels of caveolin-1, caveolin-2, annexin-1, moesin, Eph2A, and uPA were associated with in vitro sensitivity to dasatinib (Finn et al, 2007; Huang et al, 2007; Wang et al, 2007). Caveolins are membrane proteins involved in receptor-independent endocytosis and can be upregulated during epithelial-to-mesenchymal transition (Bailey and Liu, 2008). Moesin, a member of the ezrin/radixin/moesin family of actin-binding proteins, cross-links the actin cytoskeleton to the plasma membrane (Rosenblatt, 2008) and recent data suggest that cell movement through rapid actin cytoskeleton remodelling is mediated by moesin activation (Fu et al, 2008). Thus, high expression of these two markers that are involved in cell adhesion and migration may characterise a cell phenotype that is particularly susceptible to Src inhibition. Moreover, of the known dasatinib targets, increased expression of Eph2A, Yes, and Lyn was associated with in vitro sensitivity to dasatinib.
Normal epithelial architecture and function are maintained by integrins and cadherins, transmembrane receptors that mediate cell–ECM and cell–cell interactions, respectively. Importantly, signalling centres organised by these receptors modulate anchorage dependence and contact-dependent inhibition of cell growth, which are essential cellular functions often disrupted in cancer. E-cadherin is the main cell–cell adhesion molecule in epithelial tissues and is regarded as a master organiser of the epithelial phenotype. E-cadherin is regulated in part by cytoplasmic binding partners called catenins (β-catenin, and p120-catenin). β-Catenins are primarily associated with physical and/or functional linkage to the actin infrastructure (Yamada et al, 2005), whereas p120-catenin appears to modulate cadherin stability at the cell surface (Ireton et al, 2002; Reynolds and Roczniak-Ferguson, 2004). Thus, high expression of E-cadherin and p120-catenin, which are hallmarks of an epithelial cell phenotype, may characterise tumour cells that are less susceptible to Src inhibition.
Our study also confirms that low expression of IGFBP2 may be associated with in vitro sensitivity to dasatinib (Huang et al, 2007). Conversely, high expression of IGFBP2 may thus characterise a cell phenotype less responsive to the inhibition of Src- or FAK-driven transformation. Recent data support this hypothesis, in that IGFBP2 was shown to independently promote cell mobility through its interaction with integrins (Schütt et al, 2004; Wang et al, 2006). Moreover, IGFBP2 has been described to be a candidate biomarker for PTEN status and PI3K/Akt pathway activation (Mehrian-Shai et al, 2007).
Dasatinib was approved for the treatment of imatinib-resistant/intolerant patients with CML or Philadelphia chromosome-positive acute lymphoblastic leukaemia at the dosage of 70 mg twice daily. However, toxicity issues have hampered the clinical development of this compound in epithelial malignancies. Nevertheless, a phase III dose-optimisation study has recently been carried out to compare the aforementioned regimen with others, including dasatinib 100 mg once daily, in patients with CML (Tyler, 2009). The results of this study showed that there was no significant difference in efficacy between these two regimens. The safety profile was improved in the 100-mg once-daily dasatinib arm with significantly reduced frequencies of grade 3–4 thrombocytopenia and all-grade pleural effusions. The number of patients who had to discontinue, reduce, or interrupt their dosage was also less among patients taking dasatinib 100 mg once daily (Tyler, 2009).
To determine how best to use dasatinib either as a single agent or in combination with chemotherapy, we conducted a series of in vitro studies to evaluate its inhibitory effects in combination with carboplatin or paclitaxel. These preclinical studies have shown significantly enhanced activity when chemotherapy is combined with dasatinib in ovarian cancer cells. Taken together, our findings support further clinical evaluation of dasatinib as a single agent or in combination with chemotherapy in patients with ovarian cancer. Moreover, the assessment of functionally implicated response predictors in these clinical trials may help to identify the patient subgroup most likely to benefit from treatment with dasatinib.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Alam SM, Fujimoto J, Jahan I, Sato E, Tamaya T (2008) Coexpression of EphB4 and ephrinB2 in tumour advancement of ovarian cancers. Br J Cancer 98: 845–851
Aletti GD, Gallenberg MM, Cliby WA, Jatoi A, Hartmann LC (2007) Current management strategies for ovarian cancer. Mayo Clin Proc 82 (6): 751–770
Bailey KM, Liu J (2008) Caveolin-1 up-regulation during epithelial to mesenchymal transition is mediated by focal adhesion kinase. J Biol Chem 283 (20): 13714–13724
Bantscheff M, Eberhard D, Abraham Y, Bastuck S, Boesche M, Hobson S, Mathieson T, Perrin J, Raida M, Rau C, Reader V, Sweetman G, Bauer A, Bouwmeester T, Hopf C, Kruse U, Neubauer G, Ramsden N, Rick J, Kuster B, Drewes G (2007) Quantitative chemical proteomics reveals mechanisms of action of clinical ABL kinase inhibitors. Nat Biotechnol 25 (9): 1035–1044
Budde RJ, Ke S, Levin VA (1994) Activity of pp60c-src in 60 different cell lines derived from human tumors. Cancer Biochem Biophys 14 (3): 171–175
Dressman HK, Berchuck A, Chan G, Zhai J, Bild A, Sayer R, Cragun J, Clarke J, Whitaker RS, Li L, Gray J, Marks J, Ginsburg GS, Potti A, West M, Nevins JR, Lancaster JM (2007) An integrated genomic-based approach to individualized treatment of patients with advanced-stage ovarian cancer. J Clin Oncol 25 (5): 517–525
Eustace AJ, Crown J, Clynes M, O’Donovan N (2008) Preclinical evaluation of dasatinib, a potent Src kinase inhibitor, in melanoma cell lines. J Transl Med 6: 53
Fincham VJ, Frame MC (1998) The catalytic activity of Src is dispensable for translocation to focal adhesions but controls the turnover of these structures during cell motility. EMBO J 17 (1): 81–92
Finn RS, Dering J, Ginther C, Wilson CA, Glaspy P, Tchekmedyian N, Slamon DJ (2007) Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal type/‘triple-negative’ breast cancer cell lines growing in vitro. Breast Cancer Res Treat 105 (3): 319–326
Fu XD, Giretti MS, Baldacci C, Garibaldi S, Flamini M, Sanchez AM, Gadducci A, Genazzani AR, Simoncini T (2008) Extra-nuclear signaling of progesterone receptor to breast cancer cell movement and invasion through the actin cytoskeleton. PLoS ONE 3 (7): e2790
Glynn SA, Gammell P, Heenan M, O′Connor R, Liang Y, Keenan J, Clynes M (2004) A new superinvasive in vitro phenotype induced by selection of human breast carcinoma cells with the chemotherapeutic drugs paclitaxel and doxorubicin. Br J Cancer 91: 1800–1807
Huang F, Reeves K, Han X, Fairchild C, Platero S, Wong TW, Lee F, Shaw P, Clark E (2007) Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer Res 67 (5): 2226–2238
Ireton RC, Davis MA, van Hengel J, Mariner DJ, Barnes K, Thoreson MA, Anastasiadis PZ, Matrisian L, Bundy LM, Sealy L, Gilbert B, van Roy F, Reynolds AB (2002) A novel role for p120 catenin in E-cadherin function. J Cell Biol 159 (3): 465–476
Johnson FM, Saigal B, Talpaz M, Donato NJ (2005) Dasatinib (BMS-354825) tyrosine kinase inhibitor suppresses invasion and induces cell cycle arrest and apoptosis of head and neck squamous cell carcinoma and non-small cell lung cancer cells. Clin Cancer Res 11 (19): 6924–6932
Kumar SR, Masood R, Spannuth WA, Singh J, Scehnet J, Kleiber G, Jennings N, Deavers M, Krasnoperov V, Dubeau L, Weaver FA, Sood AK, Gill PS (2007) The receptor tyrosine kinase EphB4 is overexpressed in ovarian cancer, provides survival signals and predicts poor outcome. Br J Cancer 96: 1083–1091
Laird AD, Li G, Moss KG, Blake RA, Broome MA, Cherrington JM, Mendel DB (2003) Src family kinase activity is required for signal tranducer and activator of transcription 3 and focal adhesion kinase phosphorylation and vascular endothelial growth factor signaling in vivo and for anchorage-dependent and -independent growth of human tumor cells. Mol Cancer Ther 2 (5): 461–469
Landgren E, Blume-Jensen P, Courtneidge SA, Claesson-Welsh L (1995) Fibroblast growth factor receptor-1 regulation of Src family kinases. Oncogene 10 (10): 2027–2035
Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, Castaneda S, Cornelius LA, Das J, Doweyko AM, Fairchild C, Hunt JT, Inigo I, Johnston K, Kamath A, Kan D, Klei H, Marathe P, Pang S, Peterson R, Pitt S, Schieven GL, Schmidt RJ, Tokarski J, Wen ML, Wityak J, Borzilleri RM (2004) Discovery of N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl) 2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem 47 (27): 6658–6661
Mao W, Irby R, Coppola D, Fu L, Wloch M, Turner J, Yu H, Garcia R, Jove R, Yeatman TJ (1997) Activation of c-Src by receptor tyrosine kinases in human colon cancer cells with high metastatic potential. Oncogene 15 (25): 3083–3090
Mehrian-Shai R, Chen CD, Shi T, Horvath S, Nelson SF, Reichardt JK, Sawyers CL (2007) Insulin growth factor-binding protein 2 is a candidate biomarker for PTEN status and PI3K/Akt pathway activation in glioblastoma and prostate cancer. Proc Natl Acad Sci USA 104 (13): 5563–5568
Mukhopadhyay D, Tsiokas L, Zhou XM, Foster D, Brugge JS, Sukhatme VP (1995) Hypoxic induction of human vascular endothelial growth factor expression through c-Src activation. Nature 375 (6532): 577–581
Muthuswamy SK, Siegel PM, Dankort DL, Webster MA, Muller WJ (1994) Mammary tumors expressing the neu proto-oncogene possess elevated c-Src tyrosine kinase activity. Mol Cell Biol 14 (1): 735–743
Nam S, Kim D, Cheng JQ, Zhang S, Lee JH, Buettner R, Mirosevich J, Lee FY, Jove R (2005) Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res 65 (20): 9185–9189
Pegram MD, Konecny GE, O′Callaghan C, Beryt M, Pietras R, Slamon DJ (2004) Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst 96 (10): 739–749
Rahimi N, Hung W, Tremblay E, Saulnier R, Elliott B (1998) c-Src kinase activity is required for hepatocyte growth factor-induced motility and anchorage-independent growth of mammary carcinoma cells. J Biol Chem 273 (50): 33714–33721
Reynolds AB, Roczniak-Ferguson A (2004) Emerging roles for p120-catenin in cell adhesion and cancer. Oncogene 23 (48): 7947–7956
Rosenblatt J (2008) Mitosis: moesin and the importance of being round. Curr Biol 18 (7): 292–293
Schaller MD (2001) Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta 1540 (1): 1–21
Schütt BS, Langkamp M, Rauschnabel U, Ranke MB, Elmlinger MW (2004) Integrin-mediated action of insulin like growth factor binding protein-2 in tumor cells. J Mol Endocrinol 32 (3): 859–868
Serrels A, Macpherson IR, Evans TR, Lee FY, Clark EA, Sansom OJ, Ashton GH, Frame MC, Brunton VG (2006) Identification of potential biomarkers for measuring inhibition of Src kinase activity in colon cancer cells following treatment with dasatinib. Mol Cancer Ther 5 (12): 3014–3022
Song L, Morris M, Bagui T, Lee FY, Jove R, Haura EB (2006) Dasatinib (BMS-354825) selectively induces apoptosis in lung cancer cells dependent on epidermal growth factor receptor signaling for survival. Cancer Res 66 (11): 5542–5548
Sood AK, Coffin JE, Schneider GB, Fletcher MS, DeYoung BR, Gruman LM, Gershenson DM, Schaller MD, Hendrix MJ (2004) Biological significance of focal adhesion kinase in ovarian cancer: role in migration and invasion. Am J Pathol 165 (4): 1087–1095
Summy JM, Gallick GE (2003) Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev 22 (4): 337–358
Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, Cortes J, O′Brien S, Nicaise C, Bleickardt E, Blackwood-Chirchir MA, Iyer V, Chen TT, Huang F, Decillis AP, Sawyers CL (2006) Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med 354: 2531–2541
Thaker PH, Deavers M, Celestino J, Thornton A, Fletcher MS, Landen CN, Kinch MS, Kiener PA, Sood AK (2004) EphA2 expression is associated with aggressive features in ovarian carcinoma. Clin Cancer Res 10: 5145–5150
Thomas SM, Brugge JS (1997) Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 13: 513–609
Tice DA, Biscardi JS, Nickles AL, Parsons SJ (1999) Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc Natl Acad Sci USA 96 (4): 1415–1420
Tsao AS, He D, Saigal B, Liu S, Lee JJ, Bakkannagari S, Ordonez NG, Hong WK, Wistuba I, Johnson FM (2007) Inhibition of c-Src expression and activation in malignant pleural mesothelioma tissues leads to apoptosis, cell cycle arrest, and decreased migration and invasion. Mol Cancer Ther 6 (7): 1962–1972
Tyler T (2009) Once-daily dasatinib for treatment of patients with chronic myeloid leukemia. Ann Pharmacother 43 (5): 920–927
Vadlamudi RK, Sahin AA, Adam L, Wang RA, Kumar R (2003) Heregulin and HER2 signaling selectively activates c-Src phosphorylation at tyrosine 215. FEBS Lett 543 (1–3): 76–80
Wang GK, Hu L, Fuller GN, Zhang W (2006) An interaction between insulin-like growth factor-binding protein 2 (IGFBP2) and integrin alpha5 is essential for IGFBP2-induced cell mobility. J Biol Chem 281 (20): 14085–14091
Wang X, Hochhaus A, Kantarjian HM, Agrawal S, Roy A, Pfister M, Chen T, Bleickardt E, Nicaise C, Shah N (2008) Dasatinib pharmacokinetics and exposure-response (E-R): Relationship to safety and efficacy in patients (pts) with chronic myeloid leukemia (CML). J Clin Oncol 26 (May 20 suppl; abstract no. 3590)
Wang XD, Reeves K, Luo FR, Xu LA, Lee F, Clark E, Huang F (2007) Identification of candidate predictive and surrogate molecular markers for dasatinib in prostate cancer: rationale for patient selection and efficacy monitoring. Genome Biol 8 (11): R255
Wiener JR, Windham TC, Estrella VC, Parikh NU, Thall PF, Deavers MT, Bast RC, Mills GB, Gallick GE (2003) Activated SRC protein tyrosine kinase is overexpressed in late-stage human ovarian cancers. Gynecol Oncol 88 (1): 73–79
Wu Q, Suo Z, Kristensen GB, Baekelandt M, Nesland JM (2006) The prognostic impact of EphB2/B4 expression on patients with advanced ovarian carcinoma. Gynecol Oncol 102: 15–21
Wykosky J, Debinski W (2008) The EphA2 receptor and ephrinA1 ligand in solid tumors: function and therapeutic targeting. Mol Cancer Res 6 (12): 1795–1806
Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ (2005) Deconstructing the cadherin–catenin–actin complex. Cell 123 (5): 889–901
Yeatman TJ (2004) A renaissance for SRC. Nat Rev Cancer 4 (6): 470–480
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Konecny, G., Glas, R., Dering, J. et al. Activity of the multikinase inhibitor dasatinib against ovarian cancer cells. Br J Cancer 101, 1699–1708 (2009). https://doi.org/10.1038/sj.bjc.6605381
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6605381
Keywords
This article is cited by
-
Treatment for ovarian clear cell carcinoma with combined inhibition of WEE1 and ATR
Journal of Ovarian Research (2023)
-
A renaissance for YES in cancer
Oncogene (2023)
-
Integrative network analysis identifies potential targets and drugs for ovarian cancer
BMC Medical Genomics (2020)
-
MicroRNA-200 family governs ovarian inclusion cyst formation and mode of ovarian cancer spread
Oncogene (2020)
-
Modified ingenol semi-synthetic derivatives from Euphorbia tirucalli induce cytotoxicity on a large panel of human cancer cell lines
Investigational New Drugs (2019)