Abstract
The purpose of this phase II randomised trial was to determine which of two schemes, raltitrexed-irinotecan or raltitrexed-oxaliplatin, offered better activity and less toxicity in patients with advanced colorectal cancer (CRC). A total of 94 patients with previously untreated metastatic CRC were included and randomised to receive raltitrexed 3 mg m−2 followed by oxaliplatin 130 mg m−2 on day 1 (arm A), or CPT-11 350 mg m−2 followed by raltitrexed 3 mg m−2 (arm B). In both arms treatment was repeated every 3 weeks. Intent-to-treat (ITT) analysis showed an overall response rate of 46% (95% CI, 29.5–57.7%) for arm A, and 34% (95% CI, 19.8–48.4%) for arm B. Median time to progression was 8.2 months for arm A and 8.8 months for arm B. After a median follow-up of 14 months, 69% of patients included in arm A were still alive, compared to 59% of those included in arm B. Overall, 31 patients (65%) experienced some episode of toxicity in arm A and 32 patients (70%) in arm B, usually grade 1–2. The most common toxicity was hepatic, with 29 patients (60%) in arm A and 24 patients (62%) in arm B, and was grade 3–4 in four (8%) and four (9%) patients, respectively. In all, 14 patients (29%) from arm A and 24 patients (52%) from arm B had some grade of diarrhoea (P<0.03). Neurologic toxicity was observed in 31 patients (64%) in arm A, and was grade 3–4 in five patients (10%), while a cholinergic syndrome was detected in nine patients (19%) in arm B. There were no differences in haematologic toxicity. One toxic death (2%) occurred in arm A and three (6.5%) in arm B. In conclusion, both schemes have high efficacy as first-line treatment in metastatic CRC and their total toxicity levels are similar. Regimens with raltitrexed seem a reasonable alternative to fluoropyrimidines.
Similar content being viewed by others
Main
For more than 40 years, 5-fluorouracil (5FU) was the only cytotoxic agent with significant activity in advanced colorectal cancer (CRC). Recently, however, the topoisomerase I inhibitor irinotecan (CPT-11) and the platinum compound oxaliplatin have shown efficacy as second-line single-agent therapy and as first-line therapy in combination with fluoropyrimidines. In large phase III trials, irinotecan in combination with 5FU/leucovorin (LV) and oxaliplatin in combination with 5FU/LV showed superior efficacy compared with 5FU/LV alone as first-line therapy (Douillard et al, 2000; Giacchetti et al, 2000; de Gramont et al, 2000; Saltz et al, 2000; Grothey et al, 2002).
Although combined therapy has been an important development in the treatment of advanced CRC, these regimens do have some disadvantages. Firstly, their increased toxicity, particularly with the CPT-11–5FU–LV combination when 5FU is administered by bolus (Ledermann et al, 2001; Rothenberg et al, 2001). Secondly, continuous-infusion-based regimens require the use of implantable access devices and portable infusion pump, which is associated with an increased risk of infection and thromboembolism (Clark and Raffin, 1990; Prandoni and Bernardi, 1999; Verso and Agnelli, 2003). Thirdly, the inconvenience and discomfort of regular hospital visits for intravenous (i.v.) drug administration, which can have a negative effect on their quality of life.
Raltitrexed is a specific inhibitor of thymidylate synthase. This enzyme has a fundamental role in the de novo synthesis of the nucleotide thymidine triphosphate, which is essential for DNA synthesis. At a dose of 3 mg m−2, it is active in a variety of tumours such as breast, pancreatic or refractory ovarian cancers (Cunningham et al, 1994), but it is in colorectal tumours where it shows the highest activity (Van Custem et al, 2002). Several phase III studies performed in patients with advanced CRC demonstrated that response rates and survival were similar to that of the combination 5FU–LV (Cunningham et al, 1995; Cocconi et al, 1998; Maughan et al, 2002). However, in another randomised trial, a survival advantage in favour of patients treated with conventional 5FU–LV was seen even when the response rate was similar (Pazdur and Vicent, 1997).
Administration as a short 15-min i.v. infusion every 3 weeks adds value to the efficacy and toxicity profile of raltitrexed. In fact, in a study which compared the patients' preferences between raltitrexed and other 5FU-based regimens with regard to side-effects and administration attributes, 91% of the patients expressed a preference for the former treatment (Young et al, 1999).
Several studies have shown a sequence-specific synergistic cytotoxicity for the combination of raltitrexed with CPT-11 (Aschele et al, 1988), while for the combination of raltitrexed with oxaliplatin both synergistic and additive antineoplastic activities have been observed (Raymond et al, 1997).
Several phase II studies with the raltitrexed–CPT-11 combination have shown response rates of 34–46% (Carnaghi et al, 2002; Feliu et al, 2004) and median survivals of 12–15.6 months. On the other hand, with the raltitrexed–oxaliplatin combination, response rates of 41–54% and median survivals of 14.6–14.8 months have been reported (Cascinu et al, 2002; Seitz et al, 2002; Santini et al, 2004). These results are comparable to those achieved with 5FU–LV combinations with CPT-11 or oxaliplatin.
In view of these encouraging clinical data and the continuing need for active regimens with an alternative mechanism of action other than 5FU–LV, evaluation of the therapeutic potential of the combination of raltitrexed with CPT-11 or oxaliplatin seems to be of considerable interest. We thus decided to initiate a randomised phase II study in previously untreated patients with advanced CRC to determine which of two schemes, raltitrexed–CPT-11 or raltitrexed–oxaliplatin, offered higher activity and less toxicity, with a view to their selection for a subsequent phase III trial which would compare this regimen with a control arm treated with a regimen of 5FU–LV and CPT-11 or oxaliplatin.
Patients and methods
Patient population
A total of 94 patients with recurrent or metastatic CRC were included from May 2002 to December 2003. They all had at least one lesion histologically confirmed adenocarcinoma. Patients who had received prior adjuvant 5FU-based chemotherapy were eligible if they had remained free of disease for at least 6 months after completion of the adjuvant therapy. Patients with operable metastatic disease were excluded from the study. Other inclusion criteria were: (1) a performance status ⩽2, according to the Eastern Cooperative Oncology Group (ECOG) scale; (2) life expectancy of at least 3 months; (3) adequate bone marrow function, that is, a granulocyte count ⩾2 × 109 l−1 and platelets >100 × 109 l−1; (4) peripheral neuropathy (⩽grade 1, National Cancer Institute Common Toxicity Criteria (NCI-CTC) scale); (5) adequate hepatic function, that is, serum bilirubin <1.25 times the upper normal limit, glutamic oxaloacetic transaminase values (SGOT) and glutamic pyruvic transaminases (SGPT) <2.5 times the upper normal limit in the absence of hepatic metastases or <5 times the upper normal limit in the presence of metastasis; (6) adequate renal function, that is, a creatinine value ⩽1.25 times the upper normal limit and creatinine clearance >65 ml min−1.
Patients with any prior chemotherapy for advanced disease, brain or meningeal metastases, or a history of any other malignancy, were excluded, except in cases of basal cell carcinoma or in situ cervical carcinoma adequately treated. Patients provided written informed consent according to the directives of the local ethical committees.
All patients had measurable disease, as defined by the presence of at least one unidimensionally measurable lesion by computed tomography scan. Pleural effusion, ascites, osteoblastic lesions or previously irradiated lesions were not accepted as measurable disease. Patients who had received radiotherapy were eligible if there was at least one measurable lesion outside the radiation field.
Treatment plan
Chemotherapy consisted of 3-weekly courses either of raltitrexed 3 mg m−2 as a 15-min i.v. infusion followed 45 min later by oxaliplatin 130 mg m−2 on day 1, given as a 2-h infusion in 250 ml of dextrose 5% (arm A), or 3-weekly courses of CPT-11 350 mg m−2 in a 30-min i.v. infusion followed by a 15-min infusion of raltitrexed 3 mg m−2 (arm B). Antiemetic and symptomatic therapies were permitted, with the exception of any vitamin supplement containing folic acid. Patients were to be withdrawn from the study as a result of any of the following: (1) objective disease progression (according to protocol criteria); (2) unacceptable toxicity or adverse event; (3) patient unwilling or unable to continue (dropouts); (4) patient lost to follow-up; (5) investigator decision that it was in the patient's best interest not to continue.
Patients were assessed for toxicity before each course and graded according to NCI-CTC version 2. Therapy was delayed for 1 week if the neutrophil count was <1.5 × 109 l−1 or the platelet count <100 × 109 l−1, or for significantly persisting nonhaematological toxicity. Therapy was definitely discontinued if toxicity persisted after a 2-week delay. In case of grade 3 or 4 haematological toxicity, the dose of all drugs was decreased by 25 or 50%, respectively. If grade 2 or 3 diarrhoea or stomatitis occurred, the dose was reduced by 25 or 50%, respectively; grade 4 diarrhoea or stomatitis led to treatment withdrawal. The oxaliplatin dose was reduced by 25% for subsequent cycles in case of persistent (⩾14 days) or temporary (7–14 days) painful paresthesia or functional impairment. In case of persistent painful paresthesia or functional impairment, or if a patient experienced any other severe neurotoxicity despite a 25% dose attenuation, oxaliplatin was omitted in subsequent cycles. In case of the occurrence of a laryngeal spasm syndrome, the duration of the oxaliplatin infusion was increased from 2 to 6 h. In case of persistent problems, oxaliplatin was omitted. The Cockcroft–Gault formula (Cockcroft and Gault, 1976) was used to calculate creatinine clearance before each cycle. If creatinine clearance was between 55 and 65 ml min−1, the dose of raltitrexed was reduced by 25% and the next cycle given 4 weeks later. If it was between 25 and 54 ml min−1 the dose of raltitrexed was reduced by 50% and the next cycle given 4 weeks later, and if it was <25 ml min−1 the treatment was interrupted.
Pretreatment and followup studies
A diagnostic workup was performed within 3 weeks prior to the start of treatment, consisting of a complete clinical history, physical examination, performance status assessment, haematological and biochemical profiles (including CEA level), a chest X-ray and a computed tomography scan of the chest and abdomen at baseline. Additional imaging investigations were performed if clinically indicated. A computed tomography scan was repeated every three courses to assess the objective response. At the end of chemotherapy, all clinical, laboratory and imaging studies were repeated and patients underwent followup examination every 2 or 3 months until death.
Response criteria
Patients were evaluated clinically on an ITT basis at least every 3 weeks and radiographically every 9 weeks. The same evaluation modality was used throughout the study. RECIST response guidelines were used (Therasse et al, 2000), defining all responses after at least 9 weeks of therapy as follows: complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD). We defined disease control as the sum of patients achieving a CR, PR or SD. Confirmation of all responses was required after 4 weeks. Duration of response was defined as the period from the first day the criteria for CR or PR were met to the progression date. Time to tumour progression (TTP) was estimated from the dates of the first course of treatment to the first documentation of disease progression. Survival was calculated by the same method from the date of the first cycle of treatment until the date of death or last known followup.
Statistical analysis
The primary end point was response rate and the secondary objectives time to progression and toxicity. Dose intensity was calculated by dividing the total mg m−2 of drug given by the number of weeks elapsed from the beginning of therapy to the end of the last cycle.
The ‘pick the winner’ format based on the randomised phase II clinical trials approach as proposed by Simon et al (1985) was used. In this approach, an accrual of 38 patients in each arm gives a 90% chance of selecting the better treatment schedule if the difference in response rate is at least 15% and the smaller response rate is assumed to be approximately 35%. Also, 10% was added to this figure to allow for losses. If both schedules were between the pre-established ranges of response rate, the less toxic regimen would be chosen.
The Wilcoxon rank-sum method was used to compare quantitative variables, the Fisher's exact test for percentages, and the Kaplan–Meier method for survival, TTP and the duration of response.
Results
Patient characteristics
A total of 94 patients with recurrent or metastatic CRC were entered into the study and received the allocated treatment subsequent to randomisation. In all, 48 patients were randomised to arm A (oxaliplatin/raltitrexed) and 46 to arm B (irinotecan/raltitrexed). As shown in Table 1, the two groups were well matched for pretreatment characteristics. The patients' median age was 64 years, and the majority (95%) had an ECOG performance status of 0 or 1. The primary tumour was located in the colon in 58 (62%) and in the rectum in 36 (38%). Synchronous metastatic disease was observed in 48 patients (51%), in 22 of which (23%) the primary tumour was not resected. Of the remaining patients, 46 (50%) presented metastases secondary to a tumour previously removed. Of these patients, 31 (33%) had previously received adjuvant chemotherapy and 11 (12%) had received chemotherapy and radiotherapy. The liver was the predominant metastatic site (68%), and the median number of involved sites was two per patient.
Seven patients were unassessable for treatment efficacy, four in arm A (one because of severe toxicity, one due to toxic death, one moved to a different city and one due to death apparently unrelated to the neoplasia, i.e., acute myocardial infarction) and three in arm B (two due to toxic death and one because of severe toxicity). These patients were retained for the ITT analysis.
Dose intensities
Patients in arm A (oxaliplatin/raltitrexed) received a total of 303 cycles, with a median of six cycles per patient (range 1–16). Patients in arm B (irinotecan/raltitrexed) received 286 cycles, with a median of six cycles per patient (range 1–23). In seven patients (15%) from arm A and eight (17%) from arm B a delay occurred in administration of the treatment. In arm A the reasons for the delay were: elevation of transaminases in four, diarrhoea in two and thrombopenia in one, and in arm B elevation of transaminases in four, diarrhoea in two and neutropenia in two. In arm A, the median dose intensity was 41 mg m−2 week−1 for oxaliplatin and 0.95 mg m−2 week−1 for raltitrexed. These doses represent 95% of the scheduled doses both for oxaliplatin and raltitrexed. In arm B, the median dose intensity was 114 mg m−2 week−1 for irinotecan and 0.97 mg m−2 week−1 for raltitrexed. These doses represent 98% of the scheduled dose for irinotecan and 97% of the scheduled dose for raltitrexed.
Tumour response and survival
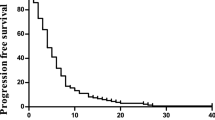
The data for response, progression-free survival and overall survival are shown in Table 2. Assessment of response was by ITT on the total population included (48 patients in arm A and 46 in arm B) and per protocol on the patients who received three or more treatment cycles and were fully assessable for response (46 in arm A and 43 in arm B). According to the ITT analysis, the overall response rate was 46% (95% CI 29.5–57.7%) for arm A (oxaliplatin/raltitrexed) and 34% (95% CI, 19.8–48.4%) for arm B (irinotecan/raltitrexed). These differences were not significant (P>0.05). In the per protocol analysis, the overall response rates were 49% (95% CI, 33.3–62.9%) and 37% (95% CI, 21.2–51.3%) (P>0.05), respectively. Median duration of response was 7.9 and 9.2 months, respectively (P=0.696, log-rank test). Overall, control of disease (CR+PR+SD) was achieved in 35 patients (69%) in arm A and in 31 patients (67%) in arm B.
At the time of performing the analysis, 37 patients (77%) in arm A and 34 patients (74%) in arm B had progressed. The median TTP was 8.2 months for arm A and 8.8 months for arm B (P=0.565, log-rank test). After a median follow-up of 14 months, 69% of the patients included in arm A were still alive, compared to 59% of those included in arm B, and therefore median survival could not be calculated.
Second-line chemotherapy with irinotecan was administered to 24 (65%) of 37 patients who progressed in arm A and second-line treatment with oxaliplatin to 21 (68%) patients who progressed in arm B.
Toxicity
Overall, 31 patients (65%) experienced some toxicity in arm A and 32 patients (70%) in arm B, usually grade 1–2. The main toxicities were gastrointestinal and haematologic. Treatment-related side effects are shown in Table 3. In general, both regimens were well-tolerated. The most common toxicity was hepatic, with elevation of transaminases being detected in 29 patients (60%) in arm A and 24 patients (62%) in arm B, although grade 3–4 toxicity was only observed in four (8%) and four (9%) of patients, respectively. It should be noted that 14 patients (31%) from arm A had some grade of diarrhoea, while it was observed in 24 patients (52%) from arm B (P<0.05). These differences can be attributed to a higher frequency of grade 1–2 diarrhoea in arm B (23 vs 39%). However, the percentage of patients with grade 3–4 diarrhoea was similar in both groups: four patients (8%) in arm A and four patients (13%) in arm B. Another very common toxicity was asthenia, usually grade 1–2. Overall, asthenia was detected in 17 patients (35%) in arm A and in 24 patients (52%) in arm B. However, these differences were not significant from a statistical point of view (P=NS). In addition, neurologic toxicity was observed in 31 patients (64%) in arm A, and was grade 3–4 in five patients (10%), while a cholinergic syndrome was detected in nine patients (19%) in arm B. One toxic death (2%) occurred in arm A, due to diarrhoea, mucositis, neutropenia and sepsis, and three (6.5%) in arm B, two due to diarrhoea, neutropenia and sepsis and one due to diarrhoea, mucositis and acute renal failure.
Discussion
In recent years, the incorporation of new drugs active against CRC with different mechanisms of action has considerably expanded the therapeutic options. However, advanced CRC continues to be an incurable disease. Therefore, the objectives of treatment should be to prolong survival and alleviate symptoms, and to improve or at least maintain the quality of life of the patients. These objectives should be achieved by administering regimens that have low toxicity, are convenient and do not require repeated visits to the hospital by the patient, so as not to worsen the patient's quality of life. The primary objective of this phase II randomised trial was to determine the activity and tolerance of two chemotherapy schemes in first-line treatment of metastatic CRC, whose main advantage over bimonthly schemes is to reduce the number of hospital visits.
The results obtained in the study suggest that both schemes have similar efficacy, with no differences being detected in either response rate (46 vs 34%) or TTP (8.2 vs 8.8 months). The response rates achieved are similar to those reported by other authors, both with schemes combining raltitrexed with oxaliplatin (39–54% response rate) (Cascinu et al, 2002; Seitz et al, 2002; Santini et al, 2004; Grávalos et al, 2005) and those combining raltitrexed with irinotecan (34–46%) (Carnaghi et al, 2002; Feliu et al, 2004). Similarly, these results are comparable to those obtained with combinations of 5FU–LV with oxaliplatin or irinotecan (Douillard et al, 2000; Giacchetti et al, 2000; de Gramont et al, 2000; Saltz et al, 2000; Grothey et al, 2002; Tournigand et al, 2004). Although it is too soon to analyse the effects on survival, there will probably not be any significant differences, since, as pointed out by several authors, what is important is to use standard drugs throughout the disease, regardless of the order in which they are used (Grothey et al, 2004; Tournigand et al, 2004), and, in our study, 64% of the patients in the raltitrexed–oxaliplatin arm and 68% of those in the raltitrexed–irinotecan arm received a second-line treatment.
As expected, the two schemes showed some differences with regard to toxicity. The raltitrexed–irinotecan scheme was associated more frequently with diarrhoea, usually grade 1–2. In addition, a higher frequency of alopecia and asthenia was observed, although the latter did not achieve statistical significance. On the other hand, the raltitrexed–oxaliplatin scheme caused neurologic toxicity in 64% of patients, although it was grade 3–4 in only 10%. However, we should note the frequency of toxic deaths observed in the study, one in the raltitrexed–oxaliplatin arm (2%) and three (6.5%) in the raltitrexed–irinotecan arm, which represents an overall rate of toxic deaths of 4.1% in the study. These results contrast with our previous experience with the raltitrexed–irinotecan scheme in a phase II trial including 91 patients in which we did not observe any toxic death (Feliu et al, 2004). On the other hand, the toxic death rate with the bimonthly continuous-infusion regimens of 5FU–LV with either oxaliplatin or irinotecan is usually <3% (Douillard et al, 2000; Giacchetti et al, 2000; de Gramont et al, 2000; Goldberg et al, 2004; Tournigand et al, 2004). However, in the joint analysis of three phase III trials comparing raltitrexed monotherapy vs 5FU–LV, a 3.8% rate of toxic deaths was observed, although 65% of these deaths can be attributed to a protocol deviation (Zalckberg, 1997). In another more recent study, which also compared raltitrexed monotherapy with other 5FU–LV schemes, a 6% rate of toxic deaths was reported in the raltitrexed arm, although in some cases treatment was administered before the patients had completely recovered from a previous toxicity (Maughan et al, 2002). In this regard, special attention should be given to the renal function of the patients before each cycle of raltitrexed to avoid the occurrence of unexpected toxicities (Jansman et al, 2000; Van Custem et al, 2002). In fact, the AUC of raltitrexed can double when creatinine clearance is less than 65 ml min−1 (Judson et al, 1998). Nevertheless, despite the fact that in our study this aspect was contemplated in the protocol and it was stressed to investigators that they should take it into account, these toxic deaths still occurred. In this regard, it has also been pointed out that patients with low serum concentrations of folate have a higher risk of toxicity from raltitrexed (Keller et al, 2001). This would be due to cells taking up and retaining raltitrexed more avidly. We do not know to what extent this aspect may have played a role in the toxicity observed in our patients.
The main advantage of combinations with raltitrexed over bimonthly schemes is the possibility of reducing the number of hospital visits. In fact, in a phase II randomised trial comparing administration of raltitrexed–oxaliplatin with the FOLFOX4 scheme, a 50% reduction was observed in the number of hospital visits with the raltitrexed regimen (Grávalos et al, 2005). Although this can currently be achieved with schemes combining oral fluoropyrimidines with oxaliplatin or irinotecan (Cassidy et al, 2004), with these regimens frequent contacts with the treatment team are required to adjust the dose or temporarily interrupt treatment in order to avoid excessive toxicity.
Based on the above, although schemes with raltitrexed have similar efficacy as that observed with 5FU–LV combinations, due to its unpredictable toxicity and the development of regimens with oral fluoropyrimidines, interest in raltitrexed has declined substantially at present. Nevertheless, it could be useful in patients in whom the use of fluoropyrimidines is contraindicated for any reason such as ischaemic heart disease. In these situations, due to its lower toxicity, administration of the combination of raltitrexed–oxaliplatin could be considered.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Aschele C, Baldo C, Sobrero AF, Debernardis D, Bornmann WG, Bertino JR (1988) Schedule-dependent synergism between raltitrexed and irinotecan in human colon cancer cells in vitro. Clin Cancer Res 4: 1323–1330
Carnaghi C, Rimassa L, Garassino I, Zucali PA, Masci G, Fallini M, Morenghi E, Santoro A (2002) Irinotecan and raltitrexed: an active combination in advanced colorectal cancer. Ann Oncol 13: 1424–1429
Cascinu S, Graziano F, Fearrau F, Catalano V, Massacesi C, Santini D, Silva RR, Barni S, Zaniboni A, Batteli N, Sierna S, Giordani P, Mari D, Baldelli AM, Antognoli S, Maisano R, Priolo D, Pessi MA, Tonini G, Rota S, Labianca R (2002) Raltitrexed plus oxaliplatin (TOMOX) as first-line chemotherapy for metastatic colorectal cancer. A phase II study of the Italian Group for the Study of Gastrointestinal Tract Carcinomas (GISCAD). Ann Oncol 13: 716–720
Cassidy J, Tabernero J, Twelves C, Brunet R, Butts C, Conroy T, Debraud F, Figer A, Grossmann J, Sawada N, Schöffski P, Sobero A, Van Cutsem E, Díaz-Rubio E (2004) XELOX (capecitabine plus oxaliplatin): active first-line therapy for patients with metastatic colorectal cancer. J Clin Oncol 22: 2084–2091
Clark DR, Raffin TA (1990) Infectious complications of indwelling long-term central venous catheters. Chest 97: 966–972
Cocconi G, Cunningham D, Van Custem E, Francois E, Gustavsson B, van Hazel G, Kerr D, Possinger K, Hietschold SM (1998) Open, randomized multicenter trial of raltitrexed vs fluorouracil plus high-dose leucovorin in patients with advanced colorectal cancer. J Clin Oncol 16: 2943–2952
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16: 31–41
Cunningham D, Zalckberg JR, Smith IE (1994) ‘Tomudex’: a novel thymidilate synthase (TS) inhibitor with clinical antitumour activity in a range of solid tumours. Ann Oncol 5 (Suppl 8): 179 (abstr 904)
Cunningham D, Zalckberg JR, Rath U, Olver I, Van Custem E, Svensson C, Seitz JF, Haper P, Kerr D, Perez-Manga G (1995) ‘Tomudex’ (ZD1694): results of a randomised trial in advanced colorectal cancer demonstrate efficacy and reduced mucositis and leucopenia. Eur J Cancer 31: 1945–1954
De Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, Braud F, Wilson C, Morvan F, Bonetti A (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18: 2938–2947
Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P (2000) Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 355: 1041–1047
Feliu J, Salud A, Escudero P, López-Gómez L, Pericay C, Castañón C, López-Tejada MR, Rodríguez-García JM, Martínez MP, Sanz M, Sánchez JJ, González-Barón M (2004) Irinotecan plus raltitrexed as first-line treatment in advanced colorectal cancer: a phase II study. Br J Cancer 90: 1502–1507
Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R, Focan C, Chollet P, Llory JF, Letourneau Y, Coudert B, Bertheaut-Cvitovich F, Larregain-Fournier D, Le Rol A, Walter S, Adam R, Misset JL, Levy F (2000) Phase III multicenter randomised trial of oxaliplatin added to chronomodulated fluorouracil–leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol 18: 136–147
Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pilot HC, Alberts SR (2004) A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 22: 23–30
Grávalos C, García-Girón C, León AI, Salud A, Esteban B, Sevilla I, Maurel J, Murias A, García-Rico E, Cortés-Funes HG (2005) TOMOX compared to FOLFOX4 as first-line treatment in patients (pts) with advanced colorectal cancer (ACRC): preliminary results of a multicenter randomized phase II trial. Proc Am Soc Clin Oncol 22: 269 (abstr 3599)
Grothey A, Deschler B, Kroening H, Ridwelski K, Reichardt P, Kretzscmar A, Clemens M, Hirschmann W, Lorenz M, Asperger W, Buechele T, Schmoll HJ (2002) Phase III study of bolus 5-fluorouracil (5-FU)/folinic acid (FA) (Mayo) vs weekly high-dose 24 h 5-FU infusion/FA+oxaliplatin (OXA) (FUFOX) in advanced colorectal cancer (ACRC). Proc Am Soc Clin Oncol 21: 129 (abstr 512)
Grothey A, Sargent D, Goldberg RM, Schmoll HJ (2004) Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil–leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 22: 1209–1214
Jansman FG, Sleijfer DT, Coenen JL, De Graaf JC, Brouwers JR (2000) Risk factors determining chemotherapeutic toxicity in patients with advanced colorectal cancer. Drug Saf 23: 255–278
Judson I, Maughan TS, Beale P, Primrose J, Hoskin P, Hawell J, Berry C, Walker M, Sutcliffe F (1998) Effects of impaired renal function on the pharmacokinetics of raltitrexed (Tomudex, ZD1694). Br J Cancer 78: 118–1193
Keller OR, Vincent M, Maroun J, Fields A, Wasil T, Moore M, Dahrouge S, Donker R, Douglas L, Abugaber AA, Donnelly J (2001) Folate depletion is associated with significant haematological toxicity in patients treated with raltitrexed (Tomudex) for colorectal cancer. Proc Am Soc Clin Oncol 20: 141 (abstr 562)
Ledermann JA, Leonard P, Seymour M (2001) Recommendation for caution with irinotecan, fluorouracil, and leucovorin for colorectal cancer. N Engl J Med 345: 145–146
Maughan TS, James RD, Kerr DJ, Lederrmann JA, McArdle C, Seymour MT, Cohen D, Hopwood P, Johnston C, Stephens RJ (2002) British MRC Colorectal Cancer Working Party. Comparison of survival, palliation, and quality of life with three chemotherapy regimens in metastatic colorectal cancer: a multicentre randomized trial. Lancet 359: 1555–1563
Pazdur R, Vicent M (1997) Raltitrexed (Tomudex) vs 5-fluorouracil and leucovorin (5FU+LV) in patients with advanced colorectal cancer (ACC): results of a randomized, multicenter, North American trial. Proc Am Soc Clin Oncol 16: 228 (abstr 801)
Prandoni P, Bernardi E (1999) Upper extremity deep vein thrombosis. Curr Opin Pulmon Med 5: 222–226
Raymond E, Buquet-Fagot C, Djelloul S, Mester J, Cvitkovic E, Allain P, Louvet C, Gespach C (1997) Antitumor activity of oxaliplatin in combination with 5-fluorouracil and the thymidine synthase inhibitor (AG 337) in human colon, breast and ovarian cancers. Anti-cancer Drugs 8: 876–885
Rothenberg ML, Meropol NJ, Poplin EA, Van Cutsem E, Wadler S (2001) Mortality associated with irinotecan plus bolus fluorouracil/leucovorin: summary findings of an independent panel. J Clin Oncol 19: 3801–3807
Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, Elfring GL, Miller LL (2000) Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med 343: 905–914
Santini D, Massacesi C, D'Agelillo RM, Marcucci F, Campisi C, Vincenzi B, Pilone A, Bianco V, Bonsignori M, Tonini G (2004) Raltitrexed plus weekly oxaliplatin as first-line chemotherapy in metastatic colorectal cancer: a multicenter non-randomized phase II study. Med Oncol 21: 59–66
Seitz JF, Bennouna J, Paillot B, Gamelin E, Francois E, Conroy T, Raoul JL, Becouarn Y, Bertheault-Cvitkovic F, Ychou M, Nasca S, Fandi a, Barthelemy P, Douillard JY (2002) Multicenter non-randomized phase II study of raltitrexed (Tomudex) and oxaliplatin in non-pretreated metastatic colorectal cancer patients. Ann Oncol 13: 1072–1079
Simon R, Wittes EE, Ellenberg SS (1985) Randomized phase II clinical trials. Cancer Treat Rep 69: 1375–1381
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, Van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205–216
Tournigand C, André T, Achille E, Lledo G, Flesh M, mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomised GERCOR study. J Clin Oncol 22: 229–237
Van Custem E, Cunningham D, Maroun J, Cervantes A, Glimelius B (2002) Raltitrexed: current clinical status and future directions. Ann Oncol 13: 513–522
Verso M, Agnelli G (2003) Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol 21: 3665–3675
Young A, Topham C, Moore J, Turner J, Wardle J, Downes M, Evans V, Kay S (1999) A patient preference study comparing raltitrexed (Tomudex) and bolus or infusional 5-fluorouracil regimens in advanced colorectal cancer: influence of side-effects and administration attributes. Eur J Cancer Care 8: 154–161
Zalckberg JR (1997) Overview of the tolerability of ‘Tomudex’ (raltitrexed): collective clinical experience in advanced colorectal cancer. Anticancer Drugs 8 (Suppl): 17–22
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Feliu, J., Castañón, C., Salud, A. et al. Phase II randomised trial of raltitrexed–oxaliplatin vs raltitrexed–irinotecan as first-line treatment in advanced colorectal cancer. Br J Cancer 93, 1230–1235 (2005). https://doi.org/10.1038/sj.bjc.6602860
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6602860
Keywords
This article is cited by
-
Comparison of irinotecan and oxaliplatin as the first-line therapies for metastatic colorectal cancer: a meta-analysis
BMC Cancer (2021)
-
Transarterial chemoembolization with raltitrexed-based or floxuridine-based chemotherapy for unresectable colorectal cancer liver metastasis
Clinical and Translational Oncology (2019)
-
Hepatic arterial chemotherapy with raltitrexed and oxaliplatin versus standard chemotherapy in unresectable liver metastases from colorectal cancer after conventional chemotherapy failure (HEARTO): a randomized phase-II study
Journal of Cancer Research and Clinical Oncology (2019)
-
Predictive factors for the development of irinotecan-related cholinergic syndrome using ordered logistic regression analysis
Medical Oncology (2018)
-
A randomized phase II study to compare oxaliplatin plus 5-fluorouracil and leucovorin (FOLFOX4) versus oxaliplatin plus raltitrexed (TOMOX) as first-line chemotherapy for advanced colorectal cancer
Clinical and Translational Oncology (2012)