Abstract
Data sources
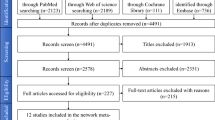
The databases searched were the Cochrane Library, PubMed/Medline, Embase, ISI Web of Knowledge, Scopus and SIGLE OpenGrey. Databases were searched with no restriction on year of publication or language.
Study selection
Randomised controlled clinical trials (RCTs), prospective and retrospective studies, case–control studies and case series studies in humans were included. The test group received implants with the local delivery of bisphosphonates, controls received implants only. Studies involving patients with metabolic bone diseases or undergoing systemic medications for bone bio-modulation or immunosuppression or with relevant pathologies were excluded.
Data extraction and synthesis
Three authors independently selected studies with disagreements being resolved by discussion. Study quality was assessed by two reviewers using the Newcastle-Ottawa scale (NOS).
Results
Three articles published between 2010 and 2013 met the selection criteria. Sample size ranged from five to 39. Mean age was 52.6 to 66. Efficacy was assessed using radiographic analysis, implant stability quotient (ISQ) and histological assessment. Despite methodological differences, all articles reported positive results for osseointegration when a local bisphosphonate was used, either on the implant surface or direct application of a pharmacologically active compound at the surgical site or in the form of a gel directly at the surgical site. Greater implant stability, better implant survival rates and reduced peri-implant bone loss were recorded in the test groups compared with controls. As well as formation of lamellar bone trabeculae in intimate contact with the implants.
Conclusions
The local use of a bisphosphonate appears to favour the osseointegration of titanium implants in humans.
Similar content being viewed by others
Commentary
Implant supported prosthesis is a valid and predictable treatment modality for missing teeth, which restores both the function and aesthetics for complete and partially edentulous patients.1,2 The success of implant placement is governed by its early osseointegration stage, which in turn is responsible for its long-term clinical success. Implant surface plays a major role in enhancing the quality of osseointegration; thus coating dental implants with bioactive materials can greatly enhance the bone healing process around the implant.2,3
Bisphosphonates are pharmacological anticatabolic agents that interfere with the normal balance of bone formation and resorption. Bisphosphonates are typically used in the treatment of systemic bone diseases such as bone tumours, osteoporosis and Paget's disease.5 It is well known that bisphosphonates have the capability to shift the balance of bone remodelling by enhancing bone formation at the expense of bone resorption through the inhibition of osteoclastic activity.3,4,5 Bisphosphonate drugs5 have several generations, where the first generation is non-nitrogen containing bisphosphonates (eg clodronate), while the second generation is nitrogen containing bisphosphonates (eg pamidronate, alendronate and ibandronate). The second generation is more potent than the first generation, hence the dose needed is way less than the first generation. Furthermore, alendronate is approximately ten times more active than pamidronate in inhibiting bone resorption.5
Bisphosphonates have a high affinity to hydroxyapatite and calcium phosphate, as well as restraining the osteoclastic activity, increasing proliferation and differentiation of osteoblasts, hence improving the osseointegration and bone formation around the implant.3,4,5,6 Inspired by this mechanism, various research groups have coated dental implants with bisphosphonates6,7 which resulted in improved implant stability, especially for patients who suffer from compromised bone quality such as diabetics and osteoporotic patients.8 Bisphosphonates can be either administrated locally or systemically. Long term systemic intake may cause complications such as medication-related osteonecrosis of the jaw (MRONJ).9 Fortunately, the low local dose of application is not enough to cause such a complication. Also, if any complication occurred it may be overlooked by removing the bone containing bisphosphonates along with the implant. Improving the quality of the bone formed around the implant may enable previously non-indicated cases to have implants placed successfully. One of these attempts is through coating the implant surface with a bioactive material.2,3,8 Among these materials is the bisphosphonates; either through applying it topically at the surgical site or through coating the implant surface with it.4
The methods of application of bisphosphonates employed among the reviewed studies have varied from one study to another. The methods included a) the local treatment of bisphosphonate through immobilising the drug on the implant surface, b) its direct application at the surgical site before surgery and c) the application of a gel infused with the drug at the implant site. The local method results have been proven by several preclinical systemic reviews.4 It is found that it does not only enhance and promote osseointegration around dental implants, but also it increases the bone implant contact (BIC).1,4 On the other hand, clinical systematic reviews are hardly achievable due to the poor standardisation in choosing the type of implant in different clinical trials.
Local administration of bisphosphonates increases the bone implant contact and implant primary stability.8 This had to be confirmed through analysing the clinical trials which used bisphosphonates.3,4 Bisphosphonates are needed in very low doses especially with the second generation, hence minimising the post-operative complications.1 Preclinical studies have proved the desirable influence of local application of bisphosphonates, however more randomised clinical studies with long follow up periods were recommended.8
Accurate clinical studies are hardly achievable due to the number of variables influencing the results, such as methodology, application technique, drug generation and dose.1,8 Despite the different methods used and site of implants . Preclinical and clinical systematic reviews studying the effect of bisphosphonates on osseointegration confirmed the efficacy of bisphosphonates in enhancing implant osseointegration.3,4,8
The current systematic review included three articles with a total of 60 cases, where each patient has received at least one bisphosphonate-treated implant (test group) and one untreated implant (control group). The authors have placed a total number of 222 implants, of which 96 were in the test group and 126 were in the control group.
The results of the review were definitive and conclusive. All articles reported positive results of osseointegration with a success rate approaching 100%. Interestingly, none of the patients suffered from any complication. Two of the review articles demonstrated excellent quality (double blinded RCT), while the third review article is a clinical trial (a pilot study for one of the other reviews). Therefore, this guaranteed applying similar methodology in two out of the three reviewed articles, which is not trivial among clinical trial due to the increased number of variables involved. The main methodology of their clinical evaluation was the radiographic assessments.
The Bisphosphonate tested local delivery system was as follows: 21/60 cases: immobiliation of second generation bisphosphonate on implant surface.
39/60 cases: implant immersion in a solution containing first generation bisphosphonate for five minutes.
All the tested implants revealed improvement in the amount of bone loss when compared to the control groups.
The implant survival rate at the five-year follow-up one of the RCTs (16/60 patients) revealed a success rate of 91.3% and 100% for the control group and the test group respectively.
Implant stability quotient was calculated in two of the studies (21/60 patients), where high stability is >70 ISQ, medium stability is between 60-69 and low stability is < 60 ISQ.10 All the tested implants revealed improvement compared to the control group. One of the studies comprised a six-month follow-up (mean±SD), where the control group: 65±6.1 and the test group: 72.6±5.4 with a difference of 6.9 units (P=0.0001). On the other hand, the other study concluded that for all implants the values ranged from 47 to 82, with a mean of 62 at insertion and 64 at abutment connection. While for the test group, the values ranged from 51 to 76, with a mean of 58 at insertion and 69 at abutment connection.
Two cases were histologically analysed showing fully osseointegrated lamellar bone trabeculae with an intimate contact to the implant.
The review searched through seven database systems and did not limit the date of publication of the study. The reviewers were not bound to the search language, which can also broaden the search limit. The author justified the low number of patients due to the limitations present and multiple variations in the delivery system of the tested drug, implant system, site of placement, evaluation criteria and difficulties in controlling the human factors.
On the other hand, two of the authors (PA and PT) of the two articles revealed conflict of interest as they have shares in a commercial company related to the coating technique. The exclusion criteria of the review excluded all patients with pathologies including diabetics and patients with osteoporosis. Though one of the main targets of bisphosphonate local delivery was to enable patients with compromised bone quality to have implants placed successfully. A sub group of patients with pathologies should have been included and reviewed. Additionally, the reviewed studies themselves only excluded uncontrolled diabetics, which does not match with the authors' exclusion criteria. The author included only individuals free of pathologies. Since there were only three studies reviewed, it would have been beneficial to review each study on its own and summarise the methods and results of each separately. For example, some studies were excluded since they did not use titanium implants, though the review inclusion criteria did not mention that implants should be made of titanium.
Practice point
-
Local administration of bisphosphonate enhances the osseointegration of implants
-
Innovative materials containing bisphosphonates could potentially empowers the hard tissue regeneration related to implant dentistry.
References
Kellesarian SV, Subhi ALHarthi S, Saleh Binshabaib M, Javed F . Effect of local zoledronate delivery on osseointegration: a systematic review of preclinical studies. Acta Odontol Scand 2017; 75:530–541.
Alghamdi HS . Methods to Improve Osseointegration of Dental Implants in Low Quality (Type-IV) Bone: An Overview. J Funct Biomater 2018; 9:pii: E7. Available from: http://www.mdpi.com/2079-4983/9/1/7 (accessed October 2018).
Aspenberg P . Bisphosphonates and implants: an overview. Acta Orthop 2009; 80:119–123.
Kellesarian SV, Abduljabbar T, Vohra F, et al. Does local ibandronate and/or pamidronate delivery enhance osseointegration? A systematic review. J Prosthodont 2018; 27:240–249.
Cha J-K, Sun Y-K, Kim MJ, Sanz M, Jung U-W . Anti-resorptive effect of pamidronate on extraction socket wall in dogs. Clin Oral Impl Res 2018; 29:688–696. https://doi.org/10.1111/clr.13260.
Abtahi J, Tengvall P, Aspenberg P . A bisphosphonate-coating improves the fixation of metal implants in human bone. A randomized trial of dental implants. Bone 2012; 50:1148–1151.Available from https://doi.org/10.1016/j.bone.2012.02.001 (accessed October 2018).
Abtahi J, Tengvall P, Aspenberg P . Bisphosphonate coating might improve fixation of dental implants in the maxilla: a pilot study. Int J Oral Maxillofac Surg 2010; 39:673–677.Available from: https://doi.org/10.1016/j.ijom.2010.04.002 (accessed October 2018).
Kellesarian SV, Abduljabbar T, Vohra F, et al. Role of local alendronate delivery on the osseointegration of implants: a systematic review and meta-analysis. Int J Oral Maxillofac Surg 2017; 46:912–921.Available from: https://doi.org/10.1016/j.ijom.2017.03.009 (accessed October 2018)
Guimarães MB, Antes TH, Dolacio MB, Pereira DD, Marquezan M . Does local delivery of bisphosphonates influence the osseointegration of titanium implants? A systematic review. Int J Oral Maxillofac Surg 2017; 46:1429–1436.
Osstell clinical guidelines for ISQ scale. Available from: https://www.osstell.com/clinical-guidelines/the-isq-scale/ (accessed October 2018).
Author information
Authors and Affiliations
Additional information
Address for correspondence: Magáli Beck Guimarãs Curso, Department of Restorative Dentistry, Faculty of Dentistry, Universidade Federal de Santa Maria, Santa Maria, RS, Brazil E-mail: magaliguimaraes@gmail.com
Guimarães MB, Antes TH, Dolacio MB, Pereira DD, Marquezan M. Does local delivery of bisphosphonates influence the osseointegration of titanium implants? A systematic review. Int J Oral Maxillofac Surg 2017; 46: 1429–1436.
Rights and permissions
About this article
Cite this article
Khamis, A., Elsharkawy, S. The influence of local delivery of bisphosphonate on osseointegration of dental implants. Evid Based Dent 19, 82–83 (2018). https://doi.org/10.1038/sj.ebd.6401326
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ebd.6401326