Abstract
Data sources
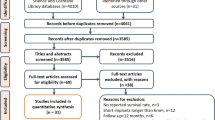
PubMed-Medline, Web of Science, Cochrane Library, ProQuest Dissertations and Theses, LILACS, Ebsco-Dentistry and Oral Sciences Source, Scirus, Embase, Scopus and Journal Ovid databases were searched. In addition hand searching of 14 relevant journals was undertaken along with screening of the reference lists of screened article and reviews.
Study selection
Randomised controlled clinical trials (RCTs), controlled clinical trials (CCTs), prospective cohort studies and case series were included. Studies where short (<10 mm) implants were placed in non-augmented healed alveolar bone with a minimum of ten implants and follow up of one year were considered
Data extraction and synthesis
One reviewer carried out the quality assessment. Implant level data were analysed. Meta-analyses were conducted and meta-regression analyses were run as fixed-effect models.
Results
Twenty-one articles reporting 16 studies (ten cohorts and six case series) were included. Seven hundred and sixty-two short implants were followed up for up to 120 months in 360 patients (mean follow-up: 44 ± 33.72 months; mean dropout rate: 5.1%). The means failure proportion (FP), biological failure proportion (BFP), prosthetic failure proportion (PFP) and radiographic marginal bone loss (MBL) were 5.9% (95% CI: 3.7–9.2%), 3.8% (95%CI: 1.9–7.4%), 2.8% (95%CI: 1.4–5.7%) and 0.83 mm (95%CI: 0.54–1.12 mm) respectively. Quantitative analysis showed that placement in the mandible (p = 0.0002) and implants with length ≤8 mm (p = 0.01) increased FP, BFP and MBL, whereas qualitative assessment revealed that crown-to-implant ratio did not influence MBL.
Conclusions
Within the limitations of the present systematic review with meta-analysis, it is suggested that single crowns supported by short implants are an acceptable and predictable option in the short- and long-term treatment of the atrophic jaws.
Similar content being viewed by others
Commentary
For many years, the use of short implants has been an area of controversy. The review in hand was an attempt to serve as an evidence base for clinical decision-making in this area.
The review asked a well-focused question. The inclusion and exclusion criteria were detailed (in fact ‘strict’ as described by the authors) and appropriate for the formulated question with the exception of including case series as explained below.
The search process and strategy were comprehensive enough to find all relevant literature. There was not any restriction on language or year of publication and unpublished data were sought.
As shown in Table 1, four outcome measures, one primary and three secondary, were identified. However, only two of the outcomes are definitively patient-oriented, FP and BFP. This raises a concern regarding the relevance of the other two outcomes to the process of clinical decision-making since only patient-oriented evidence is really of value. In addition, including disease-oriented outcomes consumes the time and effort of authors, reviewers and readers without any added clinical benefit.
Up until October 2012, only 16 studies met the review's selection criteria. None of those studies were controlled clinical trials (randomised or not). The included studies were ten cohorts and six case series. For quality assessment of the included studies, the review modified two validated tools created by the Dutch Cochrane Group (‘quality assessment of a randomized clinical trial’ and ‘quality assessment of a cohort study’) and used by Telleman et al.1
The modification was in the form of adding nine questions to each tool, raising the total number of questions from nine and eight to 18 and 17 respectively, and this resulted in a non-validated tool as mentioned by the authors in their discussion. Based on the modified tool, the authors stated that the risk of bias of the included studies was considered medium (mean 8 ± 3). It would be interesting to know what the risk of bias would be if the original tools were used and whether the modification resulted in any added value or information.
The heterogeneity of the included studies ranged from <50% to 98%. This is generally high. Among other reasons, it could be attributed to the fact that the authors combined results from different study designs, which are expected to differ systematically, resulting in increased heterogeneity.2 It would have given a better understanding of the use of short implants to conduct a separate meta-analysis for each study design although meta-analysis of case series is seldom done.
Case series are inherently biased and for the practice of health care it is still important to obtain unbiased estimates of the magnitude of large effects to make clinical and economic decisions.3 Therefore, dental clinicians cannot rely on the estimates of the effect presented in the table because they are in fact a presentation of a mixture of different study designs.
In order to be fair with the review, one needs to commend the authors for the systematic approach and comprehensiveness of reporting irrespective of the fact that PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) was not used.
In addition, the authors should be commended for their attempt to create high quality evidence for a very controversial topic. However, given the methodological issues highlighted above the results of the review are not qualified to clear the controversy.
References
Telleman G, Raghoebar GM, Vissink A, den Hartog L, Huddleston Slater JJ, Meijer HJ . A systematic review of the prognosis of short (<10 mm) dental implants placed in the partially edentulous patient. J Clin Periodontol 2011; 38: 667–676.
Higgins JPT . Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Updated March, 2011.
Reeves BC . Parachute approach to evidence based medicine: as obvious as ABC. Be Med J 2006; 333: 807–808.
Author information
Authors and Affiliations
Additional information
Address for correspondence: Luis Andre' Mezzomo, Department of Prosthodontics, Faculty of Dentistry, Pontifical Catholic University of Rio Grande do Sul, 6681 Ipiranga Avenue, 91530-001 Porto Alegre, Brazil. E-mail: lmezzomo@hotmail.com
Mezzomo LA, Miller R, Triches D, Alonso F, Shinkai RS. Meta-analysis of single crowns supported by short (<10 mm) implants in the posterior region. J Clin Periodontol 2014; 41: 191–213. doi: 10.1111/jcpe.12180. Epub 2013 Nov 25. PubMed PMID: 24266703.
Rights and permissions
About this article
Cite this article
Al-Ansari, A. Short implants supporting single crowns in atrophic jaws. Evid Based Dent 15, 85–86 (2014). https://doi.org/10.1038/sj.ebd.6401046
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ebd.6401046