Abstract
Data sources
Medline.
Study selection
Single or double blinded randomised controlled trials (RCTs), in patients suffering from orofacial pain disorders, with pain intensity as main outcome measure and antidepressants as treatment modality were included. Study quality was assessed using a 15-item checklist.
Data extraction and synthesis
Two independent investigators extracted the data and a qualitative summary was presented.
Results
Six trials were included; four studies were randomised placebo-controlled trials and two were randomised active-controlled trials. All six trials were of high quality according to the 15-item criteria. Because of the heterogeneity of treatment modalities and the low number of trials per disorder there was limited evidence to support the effectiveness of antidepressants in orofacial pain disorders.
Conclusions
More randomised controlled trials are needed to come to a firm conclusion for the use of antidepressants for orofacial pain disorders.
Similar content being viewed by others
Commentary
Orofacial pain (OFP) disorders can be life debilitating for those who suffer from chronic and acute pain conditions. Diverse pain disorders in the orofacial complex such as TMD, MFPD, neuropathic pain, atypical facial pain, cranial neuralgias, CRPS, traumatic trigeminal neuropathies, neurosensory disorders of the tongue, sleep disorders related to OFP, orofacial dystonias, neurovascular pains, headaches etc commonly seen in the head and neck region can be associated with other co-morbid conditions. The American Pain Society in 2012 reported that 100 million people in the US suffer from chronic pain.1 The prevalence studies estimate that 13 million experienced orofacial pain specifically in the US.2
Patients suffer from co-morbid psychosocial dysfunction in chronic states.3 Such co-morbidities along with varying symptomology and etiological factors, make treatment challenging for the clinician. Often interdisciplinary and multifactorial approaches must be implemented. Antidepressant therapies are being used to manage chronic pain dysfunctions and can also be beneficial in cases with co-morbid conditions.4
In the orofacial region they are used without clearly defined standardised guidelines and often combined with other therapies to reduce pain states. There are few classes of antidepressants; tricyclic antidepressant (TCA), serotonin and norepinephrine reuptake inhibitor (SNRI), serotonin selective reuptake inhibitor (SSRI), serotonin antagonist and reuptake inhibitor (SARI), used to help alleviate pain. Mechanistically they block presynaptic reuptake of 5HT serotonin, promoting pain inhibition in the CNS. There hasn't been any systematic review to date to show their effectiveness specifically in management of various orofacial pain disorders.
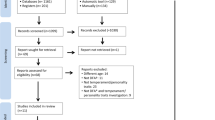
The authors of this paper sought out the evidence for the effectiveness of antidepressants on various orofacial pain disorders. Two investigators independently reviewed all trials meeting inclusionary criteria but were not blinded to outcomes of the publications in review. In disagreement, a third investigator was consulted. The authors limited their data search to Pubmed up to March 2012 and searched only in two languages, English and Dutch, for interpretation accuracy. They additionally attempted to retrieve reports from reference lists. The authors discussed that they made every attempt to retrieve published and unpublished data and disclosed that some data may have been missed. They considered both randomised placebo-controlled trials and randomised active-control trials that were sorted based on qualitative methodological scores greater than 50/100 obtained from a 15-point weighted checklist.
This careful checklist included an outline of selection and restriction criteria, treatment allocation, prognostic comparability, drop outs, interventions, co-interventions, blinding of patient/therapist/observer, outcome measures, timing of measurements, side effects analysis and presentation of data. Only those studies deemed of higher quality based on a score of 50 or higher were included in the study. Though it was a very well constructed and rigorous checklist, it is unclear how the authors allocated the division of points within each category in the checklist. It is also unclear on which standard the number 50 was chosen to be the cutoff. There is only one reference made of this methodological screening tool, utilised in a 1997 study, on lower back pain interventions.5
Six out of 142 studies methodologically qualified for inclusion in the review, with scores ranging from 52.5 to 77. Two of the studies used a cross-over design. Power of the sample sizes varied from 10-76. Mechanistically the antidepressants studied also varied; tricyclic antidepressants, serotonin selective reuptake inhibitors (SSRI), serotonin and norepinephrine reuptake inhibitors (SNRI), (SARI). The dosage frequency for each drug varied and was not made clear in the six studies. The diagnosis also varied; BMS, AFP, bruxism, TMD and radiation induced mucositis; all having different pathogeneses and pain sequelae. Criteria for diagnosis of these conditions were not identified. TMD could have implications on intracapsular derangements or masticatory musculature. Bruxism is a parafunctional habit more than a pain syndrome. Co-morbidities were not listed nor were patients' medical histories discussed. Exclusionary criteria were not defined at all in this study. Exclusion of co-morbid conditions or other pain medications that could limit the use or effectiveness of antidepressants was not mentioned at all in any of the studies.
The authors stated in a tabulated format that pain reduction was seen in 3/6 studies. They concluded for BMS trazadone was ineffective in reducing pain in a high quality study, whereas in another study paroxetine and/or sertraline showed improvement. There is limited evidence for the effectiveness of paroxetine and sertraline and ineffectiveness of trazadone in the management of BMS.
For bruxism, only one study concluded ineffectiveness of amitriptyline so evidence is limited for this. For TMD, the nature of the TMD diagnosis was not defined but only one study showed pain reduction with use of amitriptyline. There is limited evidence for its use in TMD. For head and neck cancer pain, only one study failed to show a positive effect of nortriptyline, thereby limiting evidence for its disuse in cancer pain. For atypical facial pain, only one RCT showed reduction in pain with venlafaxine, therefore limiting evidence for its use in pain management for AFP.
Comparison of the pain intensity in these studies was not possible because each had a different method of pain assessment: McGill questionnaire, VAS (visual analogue scale) and/or VRS (verbal rating scale).
The authors stated the methodological flaws of the papers reviewed as; small sample sizes, drop out biases, imprecise patient compliance, no intention-to-treat analysis, previous detail of medical history omission or lack of details of blinding methods. Only two studies demonstrated proper blinding methods.
Side effects weren't mentioned. Variation in drugs, doses, time intervals, length of treatment, sample size and diagnoses with different aetiology made this study incomparable. Meta-analysis was not done.
The investigators thoroughly disclosed biases in this study and could not exclude publication, reviewer and language biases. There may be additional studies that were not included due to the limited search strategies, using only Pubmed, limiting to two languages and reviewer bias, as they weren't blinded. They hoped to minimise this by using the 15-point checklist. It should also be noted that the authors disclosed no competing interests or funding and did not require approval from an ethical review board.
The results of this study are not enough to negate or promote the use of antidepressants in orofacial pain. No meta-analysis was conducted and so no real clinical conclusion can be reached based on variable studies in reference to all accounted biases. The need for RCTs with homogeneous studies with specific monotherapy for appropriately targeted diagnoses in orofacial pain is evident based on the search conducted by these authors. A narrower search of RCTs with clearly defined inclusion and exclusion criteria for one specific diagnosis in orofacial pain is recommended for more conclusive and comparable data analysis.
References
http://www.americanpainsociety.org/uploads/files/APS_2012_Annual_Report_FINAL.pdf. [Accessed May 19 2013].
Lipton JA, Ship JA, Larach-Robinson D . Estimated prevalence and distribution of reported orofacial pain in the United States. J Am Dent Assoc 1993; 124: 115–121.
Kroenke K, Bair MJ, Damush TM, et al. Optimized antidepressant therapy and pain self-management in primary care patients with depression and musculoskeletal pain: a randomized controlled trial. JAMA. 2009; 301: 2099–2110.
Saarto T, Wiffen PJ . Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007; 17: CD005454. Review.
Van Tulder MW, Koes BW, Bouter LM . Conservative treatment of acute and chronic nonspecific low back pain. A systematic review of randomized controlled trials of the most common interventions. Spine 1997; 22: 2128–2156.
Author information
Authors and Affiliations
Additional information
Address for correspondence: T. Forouzanfar, Department of Oral and Maxillofacial Surgery/Oral Pathology, VU University Medical Center, P.O. Box 7057, 1007 MB Amsterdam, The Netherlands. E-mail: t.forouzanfar@vumc.nl
Martin WJ, Perez RS, Tuinzing DB, Forouzanfar T. Efficacy of antidepressants on orofacial pain: a systematic review. Int J Oral Maxillofac Surg 2012; 41: 1532–1539.
Rights and permissions
About this article
Cite this article
Patel, D. Inconclusive results of a systematic review of efficacy of antidepressants on orofacial pain disorders. Evid Based Dent 14, 55–56 (2013). https://doi.org/10.1038/sj.ebd.6400938
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ebd.6400938