Abstract
Design
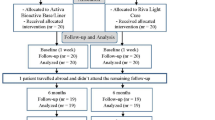
This was a randomised trial.
Intervention
Individuals who required a minimum of two replacement fillings were recruited. Restorations were placed using either Grandio bonded with Solobond M (Voco, Cuxhaven, Germany) or Tetric Ceram bonded with Syntac (Ivoclar Vivadent, Schaan, Liechtenstein).
Outcome measure
At the initial recall (baseline, ie, within 2 weeks), and after 6 months, 1 and 2 years, all restorations were assessed according to the modified United States Public Health Service (US PHS) criteria by two independent investigators using mirrors, probes, bitewing radiographs, impressions and intra-oral photographs. Recall assessments were not performed by the clinician who initially placed the restorations.
Results
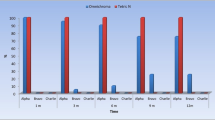
Both recall rate and survival rate were 100% after 4 years of clinical service. No significant difference was found between the restorative materials. Hypersensitivities were significantly reduced over time (P < 0.05; Friedman test). A significant deterioration over time was found for the criteria of marginal integrity (66% sufficient after 4 years),tooth integrity (15% sufficient), filling integrity (73% sufficient) and proximal contact. Stereo light microscopy and scanning electron microscopy (SEM) analysis of restoration margins revealed differences in the amount of perfect margins in favour of Tetric Ceram (P <0.05).
Conclusions
Both materials performed satisfactorily over the 4-year observation period. Because of the extension of the restorations, wear was clearly visible after 4 years of clinical service with 50% sufficient ratings.
Similar content being viewed by others
Commentary
New restorative dental materials are continually introduced to the market with claims of clinical superiority. Many of these claims are often based on early anecdotal experience and/ or the manufacturer's in-vitro mechanical testing, and not on long-term clinical studies This study compared the clinical performance of two current generational composite resins: a fine-hybrid (Tetric Ceram, 78.5% wt, 57.22% vol, 3 μm barium glass ytterbium trifluoride-Ba-Al-fluorosilicate glass, highly dispersed silicon dioxide spheroid mixed oxide) and a nanohybrid (Grandio; 87% wt, 71.4% vol, 20–15 nm silicium dioxide and glass ceramic fine particles ).1, 2, 3
Textbooks in dental materials teach that the quality (ie, particle size) and quantity (ie, weight/ volumetric percentage) of filler particles in a composite restorative material are correlated with its mechanical properties, thus inferring possible clinical performance.4, 5, 6 This study shows, however, that there was generally no significant difference between these two materials after 4 years clinical performance. By the end of the study, 86% of all restorations (n=68) were graded as at least clinically “excellent “ or “good” on six out of the nice clinical criteria. The exceptions were marginal integrity (35% “good”) and integrity of filling (1% “excellent” and 25% “good”). The authors concluded that all restorations were clinically acceptable (ie, at least “sufficient”). Although the manufacturer of the nanohybrid material funded this study, the authors candidly reported an absolute difference of 2% (P >0.05) in favour of the microhybrid in the quality of the gingival margin observed under SEM. The authors do acknowledge the weakness of assessing the marginal fit from pour-up models of teeth still in the mouth compared with extracted teeth from in-vitro studies.
The strengths of this study include: a split-mouth design, randomisation, a single operator and no-one lost to followup after 4 years. Also, only restorations with at least one gingival margin at or beyond the cemento–enamel junction were included in this study. This included teeth with amalgam replacements: typical of with what I am confronted with in clinical practice.
It is not clear, however, if the two independent observers were blinded to the type of material they were evaluating and there is no mention if they were calibrated, and no statistic (kappa value) reflecting on their observational agreement was given. The reported outcomes were also based on the US PHS scale: in 2007, dental researchers associated with the FDI World Dental Federation published recommendations for conducting controlled clinical studies of dental restorative materials,7 proposing that all future clinical research should adopt their more sensitive and, they believed, more valid scale of evaluating the clinical performance of dental restorations. The authors of this study acknowledge this issue but are excused because this trial started prior to FDI recommendations.
There are three possible reasons why the authors did not find a difference between these two restorative materials: first, there truly is no difference; second, this study did not have enough power (ie, small sample size) to show a difference (Type 2 error) or third, the US PHS scale is not sensitive enough to show a difference in this generation of vastly improved restorative materials.7 It would be interesting to test this hypothesis by comparing both the FDI and US PHS scale with this cohort during the next recall evaluation. Based on the US PHS scale, which has served clinical research for over 25 years, fine-hybrid and nanohybrid restorative materials are equally durable after 4 years of clinical service. I look forward to the published outcomes of this cohort after 8 years of service.
Practice points
-
Based on the US PHS scale, fine-hybrid and nanohybrid restorative materials are equally durable after 4 years of clinical service
References
Adabo GL, dos Santos Cruz CA, Fonseca RG, Vaz LG . The volumetric fraction of inorganic particles and the flexural strength of composites for posterior teeth. J Dent 2003 31: 353–359.
Baseren M . Surface roughness of nanofill and nanohybrid composite resin and ormocer-based tooth-colored restorative materials after several finishing and polishing procedures J Biomater Appl 2004 19: 121–134.
Curtis AR, Palin WM . The mechanical properties of nanofilled resin-based composite: characterising discrete filler particles and agglomerates using a micromanipulation technique. Dent Mater 2009 25: 180–187.
Anusavice K. Phillips'Science of Dental Materials. 11th Edn. St. Louis: Saunders; 2003.
O'Brien WJ . Dental Materials and their Selection. 4th Edn. Hanover Park: Quintessence; 2008.
Darvell DW . Materials Science for Dentistry. 9th Edn. Oxford: Woodhead; 2009.
Hickel R, Roulet JF, Bayne S, et al. Recommendations for conducting controlled clinical studies of dental restorative materials. Clin Oral Investig 2007; 11: 5–33.
Author information
Authors and Affiliations
Additional information
Address for correspondence: R. Frankenberger, Dental Clinic 1 (Operative Dentistry and Periodontology), University Medical Center Erlangen, University of Erlangen–Nuremberg, Glückstrasse 11, D-91054 Erlangen, Germany
Krämer N, Reinelt C, Richter G, Petschelt A, Frankenberger R. Nanohybrid vs. fine hybrid composite in Class II cavities: clinical results and margin analysis after four years. Dent Mater 2009; 25: 750–759.
Rights and permissions
About this article
Cite this article
Balevi, B. Fine-hybrid and nanohybrid restorative materials show similar durability in Class II cavities. Evid Based Dent 10, 107–108 (2009). https://doi.org/10.1038/sj.ebd.6400683
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ebd.6400683