Abstract
Data sources
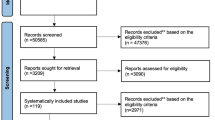
The Cochrane Pain, Palliative and Supportive Care Group Trials Register, Cochrane Central Register of Controlled Trials, Medline, Embase, LILACS (Latin American and Caribbean Health Sciences), SIGLE (System for Information on Grey Literature in Europe)(for conference proceedings) and Science Citation Index were searched, along with the reference lists of all eligible trials, key textbooks and previous systematic reviews. Authors of all identified trials were contacted.
Study selection
Studies of interest were randomised controlled trials (RCT) or quasi-RCT comparing all topical applications of lidocaine, including gels and patches in people of all ages suffering from postherpetic neuralgia (PHN; pain persisting at the site of shingles at least 1 month after the onset of the acute rash).
Data extraction and synthesis
Data were extracted independently by two authors with disputes resolved by a third reviewer. A meta-analysis was conducted using a fixed-effect approach.
Results
Three trials were included, giving a total of 182 individuals who used topical lidocaine and 132 controls. Two trials provided data on pain relief, and the remaining study provided data on secondary outcome measures. The largest trial published as an abstract compared a topical lidocaine patch to a placebo patch and accounted for 150 of the 314 patients (48%). A meta-analysis combining two of the three studies identified a significant difference between the topical lidocaine and control groups for the primary outcome measure: a mean improvement in pain relief according to a pain relief scale. Topical lidocaine relieved pain better than placebo (P 0.003). There was a statistical difference between the groups for the secondary outcome measure of mean score-reduction on a visual analogue scale (P 0.030), but this was only for a single small trial. There were a similar number of adverse skin reactions in both treatment and placebo groups.
Conclusions
There is insufficient evidence to recommend topical lidocaine as a first-line agent in the treatment of PHN with allodynia. Further research should be undertaken on the efficacy of topical lidocaine for other chronic neuropathic pain disorders, and also to compare different classes of drugs (eg, topical anaesthetics versus anti-epileptics).
Similar content being viewed by others
Commentary
PHN is defined as pain that persists following resolution of acute herpes zoster. Most often, it refers to pain that has continued after all healing has occurred and has lasted over 3 months. Up to 15% of patients with herpes zoster are likely to develop PHN and the incidence increases with age. It is most frequent in people aged 80 years and over (up to 35% of this age group are sufferers), and in this group it also tends to be more severe.
There are many trials of systemic drugs used to treat PHN with large numbers of participants, but few when it comes to local treatments. Side effects are almost inevitable with systemic drugs, so it seems logical to try using topical treatments to manage the severe allodynia which is often present and which contributes significantly to the decreased quality of life. It is disappointing to find that this Cochrane review could only identify three trials on the use of topical lidocaine, one of which is only an abstract. All the studies were carried out in the same institution and, in comparison with other PHN studies, these trials are very small. They involved only 182 patients receiving active treatment and unfortunately the largest trial was the one reported only as an abstract.1
A recent natural history study of pain following herpes zoster shows that persistent severe pain six months after the rash is rarer than previously reported and the pain does gradually decrease with time. If the pain is initially more severe then it takes longer for the pain to decline. There is only a very small sub group who continue to have clinically meaningful PHN at six months which results in decreased quality of life 2. This has important implications when interpreting clinical trials as the duration of the PHN and the time at which the patients are recruited to a trial could substantially affect results.
In the two fully reported trials in this Cochrane review, the mean duration of PHN was 36 and 48 months, which would indicate that these patients belonged to the small subset of severe PHN sufferers. These individuals still had pain over 40 mm on a scale of 0–100 mm, which is considered clinically meaningful. All the patients had allodynia.
The lignocaine patch applied in the 1996 study3 was only applied to the trunk or extremities whereas in 1995 study,4 16 had cranial PHN. It is not possible to obtain this information from the third, abstract-only, study. In the 1995 study,4 it was found that pain relief when the lidocaine was applied to the face occurred faster than when applied to other areas but it did not last as long. This could be related not only to the differing skin blood flows but also to the fact that the gel was applied under occlusive dressing in the noncranial areas. The conclusions from the review are, therefore, not entirely applicable to people who have trigeminal PHN.
The American Academy of Neurology, in its practise parameters for management of PHN published in 2004,5 use less strict criteria but suggest that topical lidocaine could be effective whereas topical capsaicin does not appear to be effective. The authors of the Cochrane Review note that the small study sizes and lack of comparison with other treatments mean topical lidocaine cannot be recommended for first-line treatment. Similar conclusions have been drawn by Wareham in the Clinical Evidence Handbook,6 where it was concluded that gabapentin and tricyclic antidepressants remain the most beneficial treatments for PHN at the present time.
Practice point
Topical lidocaine cannot be recommend as a first-line agent in the treatment of PHN: gabapentin and tricyclic antidepressants currently remain the most beneficial treatments for PHN.
References
Khaliq W, Alam S, Puri N . Topical lidocaine for the treatment of postherpetic neuralgia. Cochrane Database Syst Rev 2007; issue 2.
Thyregod HG, Rowbotham MC, Peters M, Possehn J, Berro M, Peterson KL . Natural history of pain following herpes zoster. Pain 2007; 128:148–156.
Rowbotham MC, Davies PS, Verkempinck C, Galer BS . Lidocaine patch: double-blind controlled study of a new treatments method for post herpetic neuralgia. Pain 1996; 65:39–44.
Rowbotham MC, Davies PS, Fields HL . Topical lidocaine gel relieves postherpetic neuralgia. Ann Neurol 1995; 37:246–253.
Dubinsky RM, Kabbani H, El-Chami Z, Boutwell C, Ali H . Quality Standards Subcommittee of the American Academy of Neurology practice parameter: treatment of postherpetic neuralgia: an evidence-based report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2004; 63:959–965.
Wareham D . Post-herpetic neuralgia. In Clinical Evidence Handbook. Charles Young. London: BMJ Publishing Group; 2007:pp264–265.
Author information
Authors and Affiliations
Additional information
Address for correspondence: Review Group Co-ordinator, Pain, Palliative and Supportive Care Group, Pain Research Unit, Churchill Hospital, Oxford, UK OX3 7LJ.
Khaliq W, Alam S, Puri N. Topical lidocaine for the treatment of post herpetic neuralgia. Cochrane Database Syst Rev 2007; issue 2
Rights and permissions
About this article
Cite this article
Zakrzewska, J. Insufficient evidence to recommend topical lidocaine as first-line treatment for postherpetic neuralgia. Evid Based Dent 8, 85–86 (2007). https://doi.org/10.1038/sj.ebd.6400514
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ebd.6400514