Abstract
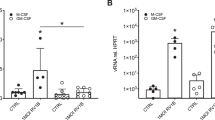
Toll-like receptors (TLRs) mediate innate immune responses to microbes. TLR2, TLR5, TLR6, and TLR9 have been implicated in responses to bacterial components, and TLR4 is the receptor for Gram-negative bacteria. Recently, TLR4 was described to function in respiratory syncytial virus-induced NF-κB activation. Here we have analyzed TLR1–9 mRNA expression in human primary macrophages infected with influenza A and Sendai viruses. TLR1, TLR2, TLR4, TLR6, and TLR8 mRNAs were expressed at basal levels in macrophages. Viral infection enhanced TLR1, TLR2, TLR3, and TLR7 mRNA expression, and neutralizing anti-IFN-α/β antibodies downregulated gene expression of these TLRs. Exogenously added IFN-α upregulated TLR1, TLR2, TLR3, and TLR7 mRNA expression in macrophages, as well as TLR3 mRNA expression in epithelial and endothelial cell lines. IFN-γ enhanced the expression of TLR1 and TLR2 mRNA in macrophages, and TLR3 in epithelial and endothelial cells. The data suggests a novel role for IFNs in the activation of innate immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Medzhitov R, Janeway C Jr Innate immunity N Engl J Med 2000 343 338–344
Takeuchi O, Hoshino K, Kawai T et al Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components Immunity 1999 11 443–451
Ozinsky A, Underhill DM, Fontenot JD et al The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors Proc Natl Acad Sci 2000 97 13766–13771
Hajjar AM, O’Mahony DS, Ozinsky A et al Functional interactions between toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin J Immunol 2001 166 15–19
Hayashi F, Smith KD, Ozinsky A et al The innate immune response to bacterial flagellin is mediated by toll-like receptor 5 Nature 2001 410 1099–1103
Hemmi H, Takeuchi O, Kawai T et al A toll-like receptor recognizes bacterial DNA Nature 2000 408 740–745
Poltorak A, He X, Smirnova I et al Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene Science 1998 282 2085–2088
Hoshino K, Takeuchi O, Kawai T et al Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product J Immunol 1999 162 3749–3752
Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction J Biol Chem 1999 274 10689–10692
Kurt-Jones EA, Popova L, Kwinn L et al Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus Nat Immunol 2000 5 398–401
Robinson D, Shibuya K, Mui A et al IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activates IRAK and NFkappaB Immunity 1997 7 571–581
Muzio M, Ni J, Feng P, Dixit VM IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling Science 1997 278 1612–1615
Wesche H, Henzel WJ, Shillinglaw W, Li S, Cao Z MyD88: an adapter that recruits IRAK to the IL-1 receptor complex Immunity 1997 7 837–847
Medzhitov R, Preston-Hurlburt P, Kopp E et al MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways Mol Cell Biol 1998 2 253–258
Burns K, Martinon F, Esslinger C et al MyD88, an adapter protein involved in interleukin-1 signaling J Biol Chem 1998 273 12203–12209
Thomassen E, Bird TA, Renshaw BR, Kennedy MK, Sims JE Binding of interleukin-18 to the interleukin-1 receptor homologous receptor IL-1Rrp1 leads to activation of signaling pathways similar to those used by interleukin-1 J Interferon Cytokine Res 1998 12 1077–1088
Adachi O, Kawai T, Takeda K et al Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function Immunity 1998 9 143–150
Kanakaraj P, Ngo K, Wu Y et al Defective interleukin (IL)-18-mediated natural killer and T helper cell type 1 responses in IL-1 receptor-associated kinase (IRAK)-deficient mice J Exp Med 1999 189 1129–1138
Medzhitov R, Preston-Hurlburt P, Janeway C A human homologue of the Drosophila toll protein signals activation of adaptive immunity Nature 1997 388 394–397
Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan FJ A family of human receptors structurally related to Drosophila Toll Proc Natl Acad Sci USA 1998 95 588–593
Chaudhary PM, Ferguson C, Nguyen V et al Cloning and characterization of two toll/interleukin-1 receptor-like genes TIL3 and TIL4: evidence for a multi-gene receptor family in humans Blood 1998 91 4020–4027
Muzio M, Natoli G, Saccani S, Levrero M, Mantovani A The human toll signaling pathway: divergence of nuclear factor κB and JNK/SAPK activation upstream of tumor necrosis factor receptor-associated factor 6 (TRAF6) J Exp Med 1998 187 2097–2101
Zhang FX, Kirschning CJ, Mancinelli R et al Bacterial lipopolysaccharide activates nuclear factor-κB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes J Biol Chem 1999 274 7611–7614
Pirhonen J, Sareneva T, Kurimoto M, Julkunen I, Matikainen S Virus infection activates IL-1β and IL-18 production in human macrophages by a caspase-1-dependent pathway J Immunol 1999 162 7322–7329
Matikainen S, Pirhonen J, Miettinen M et al Influenza A and Sendai viruses induce differential chemokine gene expression and transcription factor activation in human macrophages Virology 2000 276 138–147
Ronni T, Matikainen S, Sareneva T et al Regulation of IFN-α/β, MxA, 2′, 5′-oligoandenylate synthetase, and HLA gene expression in influenza A-infected human lung epithelial cells J Immunol 1997 158 2363–2374
Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D Recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via toll-like receptor 2 J Immunol 1999 163 1–5
Schwandner R, Dziarski R, Wesche H, Rothe M, Kirscning CJ Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2 J Biol Chem 1999 274 17406–17409
Underhill DM, Ozinsky A, Hajjar AM et al The toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens Nature 1999 401 811–815
Brightbill HD, Libarty DH, Krutzik SR et al Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors Science 1999 285 732–739
Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis J Immunol 1999 163 3920–3927
Underhill DM, Ozinsky A, Smith KD, Aderem A Toll-like receptor 2 mediates mycobacteria-induced proinflammatory signaling in macrophages Proc Natl Acad Sci 1999 25 14459–14463
Lien E, Sellati TJ, Yoshimura A et al Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products J Biol Chem 1999 247 33419–33425
Hirschfeld M, Kirschning CJ, Schwandner R et al Inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2 J Immunol 1999 163 2382–2386
Lien E, Means TK, Heine H et al Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide J Clin Invest 2000 105 497–504
Poltorak A, Ricciardi-Castagnoli P, Citterio S, Beutler B Physical contact between lipopolysaccharide and toll-like receptor 4 revealed by genetic complementation Proc Natl Acad Sci 2000 97 2163–2167
Kirschning CJ, Wesche H, Ayres TM, Rothe M Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide J Exp Med 1998 188 2091–2097
Hirschfeld M, Kirschning CJ, Schwandner R et al Inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2 J Immunol 1999 163 2382–2386
Sareneva T, Matikainen S, Kurimoto M, Julkunen I Influenza A virus-induced IFN-α/β and IL-18 synergistically enhance IFN-γ gene expression in human T cells J Immunol 1998 160 6032–6038
Matsuguchi T, Musikacharoen T, Ogawa T, Yoshikai Y Gene expressions of toll-like receptor 2, but not toll-like receptor 4, is induced by LPS and inflammatory cytokines in mouse macrophages J Immunol 2000 165 5767–5772
Musikacharoen T, Matsuguchi T, Kikuchi T, Yoshikai Y NF-κB and STAT5 play important roles in the regulation of mouse toll-like receptor 2 gene expression J Immunol 2001 166 4516–4524
Faure E, Equils O, Sieling PA et al Bacterial lipopolysaccharide activates NF-κB through toll-like receptor (TLR4) in cultured human dermal endothelial cells J Biol Chem 2000 275 11058–11063
Rehli M, Poltorak A, Schwarzfischer L, Krause SW, Andreesen R, Beutler B PU.1 and interferon consensus sequence-binding protein regulate the myeloid expression of the human toll-like receptor 4 gene J Biol Chem 2000 275 9773–9781
Beutler B, Tkacenko V, Milsark I, Krochin N, Cerami A Effect of gamma interferon on cachectin expression by mononuclear phagocytes. Reversal of the lpsd (endotoxin resistance) phenotype J Exp Med 1986 164 1791–1796
Yang R-B, Mark MR, Gray A et al Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signaling Nature 1998 395 284–288
Muzio M, Bosisio D, Polentarutti N et al Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells J Immunol 2000 164 5998–6004
Sareneva T, Julkunen I, Matikainen S IFN-α and IL-12 induce IL-18 receptor gene expression in human NK and T cells J Immunol 2000 165 1933–1938
Harroch S, Gothelf Y, Revel M, Chebath J 5′upstream sequences of MyD88, an IL-6 primary response gene in M1 cells: detection of functional IRF-1 and Stat factor binding sites Nucleic Acids Res 1995 23 3539–3546
WHO Collaborating Centers for Reference and Research of Influenza Concepts and Procedures for Laboratory-Based Influenza Surveillance. U.S. Department of Health and Human Services, National Institutes of Health, Bethesda, MD, USA 1982
Cantell K, Hirvonen S Preparation and assay of Sendai virus Methods Enzymol 1981 78 299–301
Miettinen M, Lehtonen A, Julkunen I, Matikainen S Lactobacilli and streptococci activate NF-κB and STAT signaling pathways in human macrophages J Immunol 2000 164 3733–3740
Mogensen KE, Pyhälä R, Cantell K Raising antibodies to human leukocyte interferon Acta Pathol Microbiol Scand 1975 83 443–450
Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease Biochemistry 1979 18 5294–5299
Glisin V, Crkvenjakov R, Buys C Ribonucleic acid isolated by cesium chloride centrifugation Biochemistry 1974 13 2633–2637
Miettinen M, Matikainen S, Vuopio-Varkila J et al Lactobacilli and streptococci induce interleukin-12 (IL-12), IL-18, and gamma interferon production in human peripheral blood mononuclear cells Infect Immun 1998 66 6058–6062
Takeuchi T, Kawai T, Sanjo H et al TLR6: a novel member of an expanding toll-like receptor family Gene 1999 231 59–65
Cantell K, Hirvonen S, Kauppinen M-L, Kalkkinen N Rapid production of IFN-γ in uninduced human leukocyte suspensions J Interferon Res 1991 11 231–236
Acknowledgements
We thank Mari Aaltonen, Valma Mäkinen, Teija Westerlund, and Marika Yliselä for technical assistance. Drs Osamu Takeuchi and Shizuo Akira are thanked for providing the full-length TLR6 cDNA.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the Maud Kuistila Foundation, University of Helsinki 350th Anniversary Fund, the Medical Research Council of the Academy of Finland, the Sigrud Juselius Foundation, the Jenny and Antti Wihuri Foundation, and the Finnish Cancer Foundations.
Rights and permissions
About this article
Cite this article
Miettinen, M., Sareneva, T., Julkunen, I. et al. IFNs activate toll-like receptor gene expression in viral infections . Genes Immun 2, 349–355 (2001). https://doi.org/10.1038/sj.gene.6363791
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gene.6363791
Keywords
This article is cited by
-
Integrated vector genomes may contribute to long-term expression in primate liver after AAV administration
Nature Biotechnology (2023)
-
Differences in gene regulation by TLR3 and IPS-1 signaling in murine corneal epithelial cells
Scientific Reports (2023)
-
Viral inhibitory potential of hyoscyamine in Japanese encephalitis virus–infected embryonated chicken eggs involving multiple signaling pathways
Archives of Virology (2023)
-
Pan-cancer analysis of longitudinal metastatic tumors reveals genomic alterations and immune landscape dynamics associated with pembrolizumab sensitivity
Nature Communications (2021)
-
Micronutrients and many important factors that affect the physiological functions of toll-like receptors
Bulletin of the National Research Centre (2019)