Abstract
We report on a 2-year-old girl with situs ambiguus comprising right-sided stomach and spleen, left-sided liver and complex cardiac defect. Psychomotor development of this patient was normal, and no other major abnormalities were present. Chromosome analysis revealed a de novo balanced chromosome translocation t(X;1)(q26;p13.1). Molecular cytogenetic investigations identified a breakpoint spanning BAC clone on the X-chromosome containing the ZIC3 gene. Mutations in ZIC3 are associated with situs ambiguus and cardiac defects predominantly in males. This is the first report of a live born girl with an X-autosome translocation involving the ZIC3 region.
Similar content being viewed by others
Introduction
Heterotaxy is a class of congenital malformations resulting from failed establishment of left–right asymmetry of the thoracic and visceral organs. The clinical consequences show great variability and comprise reversed (situs inversus), indeterminate (situs ambiguus) and normal position (situs solitus) of the internal organs; abnormalities of spleen (asplenia or polysplenia) and lungs (bilateral trilobed or bilobed lungs); and complex cardiovascular malformations.1 Heterotaxy has been associated with mutations in several genes,1 including ZIC3, which is located in Xq26 and encodes a zinc-finger transcription factor (MIM 306955).2, 3, 4, 5, 6 ZIC3-associated heterotaxy is an X-linked recessive disorder of variable clinical expression that predominantly affects males, but one heterozygous female with isolated congenital heart defect has also been reported.4
In females, constitutional X-autosome translocations with one breakpoint in the ZIC3 region can lead to functional nullisomy of ZIC3 by suppressed expression of the gene on the translocation chromosome (caused by disruption of the gene, or position effects) and preferential inactivation of the normal X-chromosome. This situation has recently been reported in a female foetus with a balanced translocation t(X;21)(q26;p13) and the clinical features of ZIC3 mutations (situs ambiguus and cardiac malformations).7
Here, we report on a 2-year-old girl with a chromosome breakpoint in the ZIC3 region and congenital complex heart malformation, situs ambiguus and normal psychomotor development.
Clinical report
The girl was born at term after an uneventful pregnancy (birth weight 2800 g (10th percentile), body length 47 cm (25th percentile), head circumference 32 cm (50th percentile)) to healthy and nonconsanguineous parents. Systolic murmur and cyanosis were noticed 6 days after birth. Investigations of the heart revealed a complex congenital cardiac malformation comprising double outlet right ventricle (arcus aortae and pulmonary artery arise from the enlarged right ventricle), small left ventricle, mitral valve insufficiency, common atrium with large atrial septal defect, persistent ductus arteriosus and persistent left superior vena cava. The girl also suffered from situs inversus (stomach and spleen on right side, liver on left side). No abnormalities of the spleen, the lungs or the urogenital organs were present. Low-set dysplastic ears and slight anal ectopia were additional features (Figure 1a and b). Cardiac surgery was performed twice (application of central aortopulmonic shunt, ligation of ductus arteriosus and proximal stem of pulmonary artery, Glenn anastomosis). Mental and motor development was normal. At the age of 2 years, body height was 86 cm (10th percentile), weight 11 kg (25th percentile) and head circumference 47 cm (50th percentile).
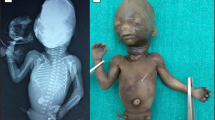
(a–d) The patient at age 2 years. Note (a) cyanosis of the lips and (b) low-set ears. (c) Partial karyogram showing G-banded homologues and ideograms of the two normal chromosomes X (red) and 1 (green), and of the translocation chromosomes. The breakpoints are in chromosome bands Xq26 and 1p13.1. (d) Hybridisation signals (red) of the breakpoint-spanning BAC RP11-478P11 on the normal chromosome X, derivative chromosome X and derivative chromosome 1.
Materials and methods
Cytogenetic investigations (GTG banding) on 25 metaphases obtained from PHA-stimulated peripheral lymphocytes were performed according to standard protocols. A de novo constitutional balanced translocation was found: 46,X,t(X;1)(q26;p13.1) (Figure 1c). The maternal and paternal karyotypes were normal.
For fluorescence in situ hybridisation (FISH) experiments, a permanent lymphoblastoid cell line of the patient was established by EBV transformation according to standard protocols after informed consent. FISH was performed using YAC clones selected from the Whitehead Institute and BAC clones from the UCSC Golden Path Genome Browser (Supplementary Table 1). DNA samples were prepared according to standard protocols and were labelled by nick translation with either biotin-16-dUTP or digoxigenin-11-dUTP. Immunocytochemical detection of probes was performed as described elsewhere.8 Chromosomes were counterstained with 46-diamino-2-phenyl-indole. Metaphases were analysed with a Zeiss epifluorescence microscope.
An X-chromosome inactivation analysis was performed at the androgen receptor locus as described previously.9
For Southern blot analysis, genomic DNA from the patient and control DNA were digested with restriction enzymes AccI, BamHI, EcoRI, HincII, PvuII and BclI, and size separated in 0.7% agarose gels. The separated DNA fragments were blotted onto nylon membranes and hybridised with [α-32P]dCTP-labelled random hexamer-primed PCR products. Five probes were employed for Southern blot analysis. The probes were generated using the following primer pairs: ZIC3_A (5′-TCCTTGGAGGGACCTTTTTC-3′; 5′-TCCTTTC-CTGCTGCAACATA-3′; product size 467 bp); ZIC3_B (5′-GCAAGTCTTTCAAGGCGAAG-3′; 5′-TTCACGGTAACCCAGAAAGG-3′; product size 446 bp); ZIC3_C (5′-GTCAATTCTCTCGCGGTCTC-3′; 5′-GCGCTAAAAACCCACTTCAA-3′; product size 501 bp); ZIC3_D (5′-ATGCCAAAAGTCCTCCTTCA-3′; 5′-AGTTGGCTCACCGAACACTT-3′; product size 546 bp); and ZIC3_E (5′-CACAGTGGTTACCCGATTCC-3′; 5′-CCATTAAGCAGATGCGTTTTC-3′; product size 449 bp).
Results
Molecular cytogenetic analysis using FISH localised the X-chromosomal breakpoint between YAC clones 943D11 and ICRFy900D5032. Successive hybridisations with smaller clones showed that BAC RP11-478P11 overlaps the breakpoint (hybridisation signals on the normal chromosome X, the derivative chromosome X and the derivative chromosome 1) (Figure 1d). The breakpoint on chromosome 1 was determined to lie between the adjacent clones BAC RP11-328E11 and BAC RP11-320J21 close to the centromere (Supplementary Table 1). The region covered by these two BAC clones on chromosome 1 contains several uncharacterised transcripts, but no known genes.
X-chromosome inactivation analysis revealed complete skewing (data not shown).
BAC RP11-478P11 on the X-chromosome includes ZIC3, but no other known genes. Southern blot analysis covering ZIC3 and approximately 20 kb on each the 5′ and the 3′ side of ZIC3 did not reveal aberrant bands in patient DNA (data not shown), leading to the conclusion that the breakpoint is neither within nor in the immediate vicinity of ZIC3. Thus, the chromosome breakpoint is located either at the 5′ or at the 3′side of ZIC3 with a distance of at least 20 kb.
Discussion
It is well established that in approximately 80% of female patients with X-autosome translocations, the rearranged X-chromosome is preferentially active and the normal X-chromosome inactivated. 10, 11 This was also the case in the foetus reported by Fritz et al.7 Skewed X-inactivation was also present in our patient, thus mimicking the situation in hemizygous males (functional nullisomy). The specific phenotype of our patient is very likely caused by the absence of functional ZIC3, probably owing to disruption of the transcriptional unit from a regulatory element or owing to a position effect associated with the translocation breakpoints.
Our patient is only the second reported case of a female translocation carrier with a breakpoint in the ZIC3 region suffering from situs ambiguus and complex cardiac malformation.7 In contrast to our patient, the previously reported foetus with an X;21 translocation had asplenia, bilaterally four-lobed lungs and sacral hypoplasia as additional features. The parents of this foetus decided to terminate the pregnancy. The more complex phenotype in this foetus compared to our patient might be due to different breakpoints, possibly resulting in some residual ZIC3 expression of different degree in the two cases. On the other hand, variable phenotypes have also been described in males with identical mutations in ZIC3.2, 3, 4 The clinical features ranged from incomplete penetrance3 (i.e. no detectable malformations in spite of a truncating ZIC3 mutation, which is associated with cardiac malformations in other male family members) and cases with isolated cardiac malformations to patients with heart defects and malposition of the abdominal organs, abnormalities of the lung, the spleen, the urogenital organs, anal and vertebral anomalies, olfactory nerve aplasia, cerebellar hypoplasia, craniosynostosis, duodenal atresia, omphalocele, cystic hygroma and cholestasis.2, 3, 4 Mental deficiencies have not been reported, but many patients died at too young an age for such problems to be noticed.
References
Zhu L, Belmont JW, Ware SM : Genetics of human heterotaxias. Eur J Hum Genet 2006; 14: 17–25.
Gebbia M, Ferrero GB, Pilia G et al: X-linked situs abnormalities result from mutations in ZIC3. Nat Genet 1997; 17: 305–308.
Megarbane A, Salem N, Stephan E et al: X-linked transposition of the great arteries and incomplete penetrance among males with a nonsense mutation in ZIC3. Eur J Hum Genet 2000; 8: 704–708.
Ware SM, Peng J, Zhu L et al: Identification and functional analysis of ZIC3 mutations in heterotaxy and related congenital heart defects. Am J Hum Genet 2004; 74: 93–105.
Ferrero GB, Gebbia M, Pilia G et al: A submicroscopic deletion in Xq26 associated with familial situs ambiguus. Am J Hum Genet 1997; 61: 395–401.
Casey B, Devoto M, Jones KL, Ballabio A : Mapping a gene for familial situs abnormalities to human chromosome Xq24−q27.1. Nat Genet 1993; 5: 403–407.
Fritz B, Kunz J, Knudsen GP et al: Situs ambiguus in a female foetus with balanced (X;21) translocation – evidence for functional nullisomy of the ZIC3 gene? Eur J Hum Genet 2005; 13: 34–40.
Wirth J, Nothwang HG, van der Maarel S et al: Systematic characterisation of disease associated balanced chromosome rearrangements by FISH: cytogenetically and genetically anchored YACs identify microdeletions and candidate regions for mental retardation genes. J Med Genet 1999; 36: 271–278.
Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW : Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet 1992; 51: 1229–1239.
Schmidt M, Du Sart D : Functional disomies of the X chromosome influence the cell selection and hence the X inactivation pattern in females with balanced X-autosome translocations: a review of 122 cases. Am J Med Genet 1992; 42: 161–169.
Sharp AJ, Spotswood HT, Robinson DO, Turner BM, Jacobs PA : Molecular and cytogenetic analysis of the spreading of X inactivation in X; autosome translocations. Hum Mol Genet 2002; 11: 3145–3156.
Acknowledgements
We thank the parents of the patient for their support and Petra Viertel, Hannelore Madle and Susanne Freier for technical assistance. We gratefully acknowledge support from the German National Genome Research Network (NGFN, project number 01GR0105).
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic database information
Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/
Genome Browser, http://www.genome.ucsc.edu/cgi-bin/hgGateway
Whitehead Institute, http://www-genome.wi.mit.edu/cgi-bin/contig/phys_map
Supplementary Information accompanies the paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary information
Rights and permissions
About this article
Cite this article
Tzschach, A., Hoeltzenbein, M., Hoffmann, K. et al. Heterotaxy and cardiac defect in a girl with chromosome translocation t(X;1)(q26;p13.1) and involvement of ZIC3. Eur J Hum Genet 14, 1317–1320 (2006). https://doi.org/10.1038/sj.ejhg.5201707
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejhg.5201707