Abstract
Partial absence of the sacrum is a rare congenital defect that also occurs as an autosomal-dominant trait, whereas imperforate/ectopic anus is a relatively common malformation, usually observed in multiple congenital anomalies syndromes. We report on a girl born to healthy consanguineous parents (first cousins once removed) with anal imperforation and associated rectovaginal fistula and partial sacral agenesis. Facial dysmorphism included a high forehead, epicanthic folds, downslanting palpebral fissures, hypertelorism and a depressed nasal root. Brain MRI showed a bilateral opercular dysplasia with a unilateral (right) pachygyria; MRI and X-ray imaging of the spine disclosed a tethered cord associated with partial sacral agenesis. She showed a moderate developmental delay. Ophthalmologic examination evidenced bilateral microphthalmos and relative microcornea. Cytogenetic studies in our patient disclosed a pure de novo 6q25.3 → qter deletion. By genotype analysis, we detected in our patient a maternal allele loss encompassing D6S363 and D6S446. Pure distal 6q deletion is a rare anomaly, reported in association with sacral/anorectal malformations (sacral agenesis, anal imperforation/ectopia) and never with cortical dysplasia. Pooling deletion mapping information in patients with pure terminal and interstitial 6q deletion allowed us to define a critical region spanning 0.3 Mb between the markers D6S959 and D6S437 for sacral/anal malformations. We hypothesize that haploinsufficiency for a gene within the deleted region may impair normal development of caudal structures, possibly acting on the notochordal development.
Similar content being viewed by others
Introduction
Terminal deletions of 6q (6q25 → 6qter) have been rarely reported in the literature and have been associated to a specific phenotype. Findings in a group of 26 patients1 included mental retardation (100%), ear anomalies (88%), hypotonia (86%), microcephaly (82%), limb anomalies (71%), brain anomalies (67%), eye anomalies (50%), cardiac defects (48%), genital anomalies (48%) and seizures (38%). However, only in the patient by McLeod et al,2 a pure deletion was found. To date, subtelomeric deletion of 6q have been reported in three patients,3, 4, 5 and patients carrying a pure terminal or interstitial deletion of 6q have been rarely observed.6, 7, 8, 9, 10, 11, 12
Partial absence of the sacrum is a rare congenital defect, which also occurs as an autosomal-dominant trait; association with anterior meningocoele, presacral teratoma and anorectal abnormalities constitutes the Currarino triad (MIM 176450).
Imperforate/ectopic anus is a relatively common malformation. It has been rarely reported in familial cases (MIM 207500, MIM 301800), but no mapping data are available.
Patient report
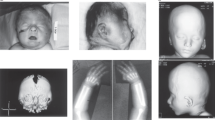
The girl was born at 35 weeks of gestation to healthy consanguineous Malagasi parents (first cousins once removed). Family history and gestation were unremarkable. Birth weight was 2500 g (10th–25th centile), length was 48 cm (25th–50th centile) and cranial circumference was 32 cm (5th–10th centile). She was immediately admitted to a tertiary care center for surgical correction of an anal imperforation with an associated rectovaginal fistula. Imaging studies showed partial sacral agenesis and a tethered cord. Echocardiographic evaluation revealed atrial and ventricular septal defects that were corrected at the age of 12 months. Clinical evaluation at 24 months showed a severe developmental delay, microcephaly, short stature and minor anomalies. Weight was 10 kg (−1.3 SD), height was 80 cm (−1.4 SD) and head circumference was 44 cm (−2.8 SD). Facial dysmorphism included a high forehead, epicanthal folds, downslanting palpebral fissures, hypertelorism and a depressed nasal root (Figure 1a). Brain MRI showed a bilateral opercular dysplasia with a unilateral (right) pachygyria (Figure 1b and c); MRI and X-ray imaging of the spine disclosed a tethered cord associated with a partial sacral agenesis (Figure 1d).
Clinical and neuroradiological aspects. (a) Facial appearance at age 3. (b) MRI: coronal section (T2), note normal gyral pattern. (c) MRI: frontal section (T1), bilateral opercular dysplasia and unilateral (right) pachygyria. (d) Sagittal MRI section of dorso-lumbar spinal canal disclosing tethered spinal cord.
At 3 years, 2/12 of age ophthalmologic examination evidenced bilateral microphthalmos (diameter of the globe <20 mm) and relative microcornea. Visual-evoked potentials and electroretinogram were normal.
Methods
Cytogenetic analysis
Subtelomeric FISH probing was performed with Cytocell Chromoprobe Multiprobe kit.
Genotype analysis
DNA from leukocytes of patient and their parents were used for genotyping. Simple fluorescent PCR assays were performed using polymorphic markers: D6S292, D6S308, D6S441, D6S1577, D6S415, D6S959, D6S363, D6S437, D6S1614, D6S1581, D6S264 and D6S446 (http://www.gdb.org/). PCR reactions were performed following standard procedures. After denaturation, each sample was loaded for electrophoresis on an Applied Biosystems model 3100 automated sequencer (PE Applied Biosystems, Perkin-Elmer). Data were analyzed using the Gene Scanner Model 3.7 Fluorescent Fragment Analyzer (PE Applied Biosystems, Perkin-Elmer) and electropherograms were generated for each sample. The samples from the patient and from his parents were processed for each marker. Data were analyzed using the Gene Scanner Model 3.7 Fluorescent Fragment Analyzer (PE Applied Biosystems, Perkin-Elmer).
Results
Metaphase cells analyzed from cultures of peripheral blood on the patient revealed a normal female chromosome complement at the 650-band level. A terminal 6q deletion was found by subtelomeric FISH. The patient's karyotype was designated as 46, XX, del (6)(q25.3qter). In both parents, FISH using identical probe for the subtelomeric region of 6p and 6q yielded normal result. There was no evidence for a balanced rearrangement in the parents. By genotype analysis, we detected in our patient a maternal allele loss for the D6S363, D6S1581, D6S264 and D6S446 markers (Figure 2).
(a) Microsatelitte analysis of the deletion. Dotted arrow is used for noninformative markers. (b) Comparative deletion mapping in patients with 6q deletion and anorectal malformations. The overlapping segment between Pirola's case and our patient is depicted in the bottom line, with the four known genes.
Discussion
Present patient shows a multiple congenital anomalies syndrome owing to a pure de novo 6q23qter deletion. A peculiar finding is the presence of a bilateral opercular dysplasia associated to a unilateral pachygyria at the brain MRI. These brain malformations are probably consequent to the chromosomal deletion, although they have never been reported to date in other patients who carry an isolate interstitial or terminal 6q25 deletion. Moreover, no locus for brain malformation has been mapped in this region, with the exception of corpus callosum agenesis (6q25). Because of the presence of parental consanguinity, a recessive phenotype compounded by a homozygote mutation is also possible. A long-range effect of the deletion on gene expression outwith the deletion is another possibility to be considered.
On the other hand, distal 6q deletions have been frequently reported in association with sacral/anorectal malformations (sacral agenesis, anal imperforation/ectopia).
Our patient showed anal imperforation with associated rectovaginal fistula and partial sacral agenesis. Probably, a common pathogenetic mechanism is involved in producing both malformations. At an early stage of development, the notochord is known to organize normal development of central axial structures, such as the spinal cord, vertebral column and anorectum. However, its role has not been completely elucidated.13 Recently, Qi et al14 suggested that an alteration in sonic hedgehog signaling may be pivotal in producing abnormal notochord development and consequently sacral/anorectal malformations.
By comparing the reported cases of affected patients carrying a pure deletion, we delimited a critical region of 0.3 Mb for sacral/anorectal malformations, lying between the markers D6S959 and D6S437 (see Table 1). The patient by Pirola et al10 shows an ectopic anus, which can be considered as a mild form of anorectal malformation. Our patient's contribution to the definition of a critical region should be cautiously considered, because of the simultaneous presence of another midline defect (agenesis of the corpus callosum).
We hypothesize that there is a gene in the deleted region whose haploinsufficiency impairs the normal development of these structures, possibly acting on the notochordal development or interfering with SHH signaling.
To date, four genes are positioned in the deleted region: SYNJ2 (synaptojanin 2), SERAC1 (serine-active site containing 1), GTF2H5 (general transcription factor iih, polypeptide 5) and TULP4 (Tubby-like protein 4) (UCSC Genome Browser, http://genome.ucsc.edu/ and Ensembl Genome Browser, http://www.ensembl.org). GTF2H5 mutations are responsible for trichothiodystrophy group A, a DNA repair syndrome, and for a form of ichthyosiform erythroderma with hair abnormality, and mental and growth retardation.15 Sacral/anorectal malformations have not been observed in the reported patients. The Synj2b protein isoforms are localized in nerve terminals in rat brain and at spermatid manchette in rat testis. In glioblastoma cell lines, Synj2b seems implicated in the regulation of the formation of invadopodia and lamellipodia.16 Mutations in SERAC1 or SYNJ2 cause male mouse sterility.17 TULP4 is a putative transcription factor of unknown function. No role has been attributed for the latter three genes in human developmental anomalies and/or diseases.
Other genes mapping outside the reported critical region could be involved, because of possible modifications on gene expression.
Report of further patients is needed to evaluate these genes as candidates in sacral/anorectal malformations, and their hypothetical role in notochordal development.
References
Hopkin RJ, Schorry E, Bofinger M et al: New insights into the phenotypes of 6q deletions. Am J Med Genet 1997; 70: 377–386.
McLeod DR, Fowlow SB, Robertson A et al: Chromosome 6q deletions: a report of two additional cases and a review of the literature. Am J Med Genet 1990; 35: 79–84.
Lorda-Sanchez I, Lopez-Pajares I, Roche MC et al: Cryptic 6q subtelomeric deletion associated with a paracentric inversion in a mildly retarded child. Am J Med Genet 2000; 95: 336–338.
Anderlid BM, Schoumans J, Anneren G et al: FISH-mapping of a 100-kb terminal 22q13 deletion. Hum Genet 2002; 110: 439–443.
Stevenson DA, Brothman AR, Carey JC et al: 6q subtelomeric deletion: is there a recognizable syndrome? Clin Dysmorphol 2004; 13: 103–106.
Narahara K, Tsuji K, Yokoyama Y et al: Specification of small distal 6q deletions in two patients by gene dosage and in situ hybridization study of plasminogen and alpha-L-fucosidase 2. Am J Med Genet 1991; 40: 348–353.
Meng J, Fujita H, Nagahara N et al: Two patients with chromosome 6q terminal deletions with breakpoints at q24.3 and q25.3. Am J Med Genet 1992; 43: 747–750.
Evers LJ, Schrander-Stumpel CT, Engelen JJ et al: Deletion of the long arm of chromosome 6: two new patients and literature review. Clin Genet 1996; 50: 138–144.
Rubtsov N, Senger G, Kuzcera H et al: Interstitial deletion of chromosome 6q: precise definition of the breakpoints by microdissection, DNA amplification, and reverse painting. Hum Genet 1996; 97: 705–709.
Pirola B, Bortotto L, Giglio S et al: Agenesis of the corpus callosum with Probst bundles owing to haploinsufficiency for a gene in an 8 cM region of 6q25. J Med Genet 1998; 35: 1031–1033.
Sukumar S, Wang S, Hoang K et al: Subtle overlapping deletions in the terminal region of chromosome 6q24.2–q26: three cases studied using FISH. Am J Med Genet 1999; 87: 17–22.
Lukusa T, Willekens D, Lukusa N et al: Terminal 6q25.3 deletion and abnormal behaviour. Genet Counsel 2001; 12: 213–221.
Stemple DL : Structure and function of the notochord: an essential organ for chordate development. Development 2005; 132: 2503–2512.
Qi BQ, Beasley SW, Frizelle FA : Evidence that the notochord may be pivotal in the development of sacral and anorectal malformations. J Pediatr Surg 2003; 38: 1310–1316.
Giglia-Mari G, Coin F, Ranish JA et al: A new, tenth subunit of TFIIH is responsible for the DNA repair syndrome trichothiodystrophy group A. Nat Genet 2004; 36: 714–719.
Chuang YY, Tran NL, Rusk N et al: Role of synaptojanin 2 in glioma cell migration and invasion. Cancer Res 2004; 64: 8271–8275.
Schimenti JC, Reynolds JL, Planchart A : Mutations in Serac1 or Synj2 cause proximal t haplotype-mediated male mouse sterility but not transmission ratio distortion. Proc Natl Acad Sci USA 2005; 102: 3342–3347.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Titomanlio, L., Giurgea, I., Baumann, C. et al. A locus for sacral/anorectal malformations maps to 6q25.3 in a 0.3 Mb interval region. Eur J Hum Genet 14, 971–974 (2006). https://doi.org/10.1038/sj.ejhg.5201635
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejhg.5201635
Keywords
This article is cited by
-
Currarino syndrome: a comprehensive genetic review of a rare congenital disorder
Orphanet Journal of Rare Diseases (2021)
-
Interstitial deletion of 6q25.2–q25.3: a novel microdeletion syndrome associated with microcephaly, developmental delay, dysmorphic features and hearing loss
European Journal of Human Genetics (2009)