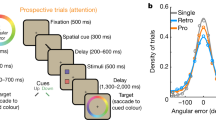
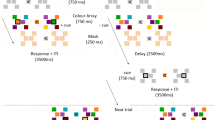
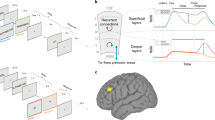
Abstract
Knowledge or experience is voluntarily recalled from memory by reactivation of the neural representations in the cerebral association cortex1,2,3,4. In inferior temporal cortex, which serves as the storehouse of visual long-term memory5,6,7,8, activation of mnemonic engrams through electric stimulation results in imagery recall in humans9, and neurons can be dynamically activated by the necessity for memory recall in monkeys10,11. Neuropsychological studies12 and previous split-brain experiments13 predicted that prefrontal cortex exerts executive control upon inferior temporal cortex in memory retrieval; however, no neuronal correlate of this process has ever been detected. Here we show evidence of the top-down signal from prefrontal cortex. In the absence of bottom-up visual inputs, single inferior temporal neurons were activated by the top-down signal, which conveyed information on semantic categorization imposed by visual stimulus–stimulus association. Behavioural performance was severely impaired with loss of the top-down signal. Control experiments confirmed that the signal was transmitted not through a subcortical but through a fronto-temporal cortical pathway. Thus, feedback projections from prefrontal cortex to the posterior association cortex2,3,14 appear to serve the executive control of voluntary recall.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Goldman-Rakic,P. S. in Handbook of Physiology Vol. 5 (ed. Plum, F.) 373–417 (American Physiological Society, Bethesda, 1987).
Fuster,J. M. The Prefrontal Cortex: Anatomy, Physiology, and Neuropsychology of the Frontal Lobe 3rd edn (Lippincott-Raven, Philadelphia, 1997).
Petrides,M. in Handbook of Neuropsychology (eds Boller, F. & Grafman, J.) 9, 59–82 (Elsevier, Amsterdam, 1994).
Miyashita,Y. in The Cognitive Neurosciences 2nd edn (ed. Gazzaniga, M. S.) in the press (MIT Press, Cambridge, MA).
Mishkin,M. A memory system in the monkey. Phil. Trans. R. Soc. Lond. B 298, 83–95 (1982).
Rolls,E. T. Neural organization of higher visual functions. Curr. Opin. Neurobiol. 1, 274–278 (1991).
Miyashita,Y. Inferior temporal cortex: where visual perception meets memory. Annu. Rev. Neurosci. 16, 245–263 (1993).
Desimone,R. & Duncan,J. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 18, 193–222 (1995).
Penfield,W. & Perot,P. The brain's record of auditory and visual experience. Brain 86, 595–696 (1963).
Sakai,K. & Miyashita,Y. Neural organization for the long-term memory of paired associates. Nature 354, 152–155 (1991).
Naya,Y., Sakai,K. & Miyashita,Y. Activity of primate inferotemporal neurons related to a sought target in pair-association task. Proc. Natl Acad. Sci. USA 93, 2664–2669 (1996).
Gazzaniga,M. S. Principles of human brain organization derived from split-brain studies. Neuron 14, 217–228 (1995).
Hasegawa,I., Fukushima,T., Ihara,T. & Miyashita,Y. Callosal window between prefrontal cortices: cognitive interaction to retrieve long-term memory. Science 281, 814–818 (1998).
Chafee,M. V. & Goldman-Rakic,P. S. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J. Neurophysiol. 79, 2919–2940 (1998).
Eacott,M. J. & Gaffan,D. Interhemispheric transfer of visual learning in monkeys with intact optic chiasm. Exp. Brain Res. 74, 348–352 (1989).
Sidtis,J. J., Volpe,B. T., Holtzman,J. D., Wilson,D. H. & Gazzaniga,M. S. Cognitive interaction after staged callosal section: evidence for transfer of semantic activation. Science 212, 344–346 (1981).
Gross,C. G., Bender,D. B. & Mishkin,M. Contributions of the corpus callosum and the anterior commissure to visual activation of inferior temporal neurons. J. Neurophysiol. 131, 227–239 (1977).
Doty,R. W., Ringo,J. L. & Lewine,J. D. Forebrain commissures and visual memory: a new approach. Behav. Brain Res. 29, 267–280 (1988).
Felleman,D. J. & Van Essen,D. C. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex 1, 1–47 (1991).
Ringo,J. L. & O'Neill,S. G. Indirect inputs to ventral temporal cortex of monkey: the influence of unit activity of alerting auditory input, interhemispheric subcortical visual input, reward, and the behavioral response. J. Neurophysiol. 70, 2215–2225 (1993).
Rainer,G., Rao,S. C. & Miller,E. K. Prospective coding for objects in primate prefrontal cortex. J. Neurosci. 19, 5493–5505 (1999).
Goldman-Rakic,P. S. Regional and cellular fractionation of working memory. Proc. Natl Acad. Sci. USA 93, 13473–13480 (1996).
Buckner,R. L., Raichle,M. E., Miezin,F. M. & Petersen,S. E. Functional anatomic studies of memory retrieval for auditory words and visual pictures. J. Neurosci. 16, 6219–6235 (1996).
Fletcher,P. C., Shallice,T., Frith,C. D., Frackowiak,R. S. & Dolan,R. J. The functional roles of prefrontal cortex in episodic memory. II. Retrieval. Brain 121, 1249–1256 (1998).
Eacott,M. J. & Gaffan,D. Inferotemporal-frontal disconnection: the uncinatee fascicle and visual associative learning in monkeys. Eur. J. Neurosci. 4, 1320–1332 (1992).
Gutnikov,S. A., Ma,Y. & Gaffan,D. Temporo-frontal disconnection impairs visual–visual paired association learning but not configural learning in Macaca monkeys. Eur. J. Neurosci. 9, 1524–1529 (1997).
Fuster,J. M., Bauer,R. H. & Jervey,J. P. Functional interactions between inferotemporal and prefrontal cortex in a cognitive task. Brain Res. 330, 299–307 (1985).
MacPherson,J. A. & Aldridge,J. W. A quantitative method of computer analysis of spike train data collected from behaving animals. Brain Res. 175, 183–187 (1979).
Sary,G., Vogels,R. & Orban,G. A. Cue-invariant shape selectivity of macaque inferior temporal neurons. Science 260, 995–997 (1993).
Acknowledgements
We thank Y. Naya for technical advice. This work was supported by a grant-in-aid for Specially Promoted Research from the Ministry for Education, Science and Culture of Japan, a grant from the Magnetic Health Science Foundation (Y.M.) and a grant from the Ministry for Education, Science and Culture of Japan (I.H.).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tomita, H., Ohbayashi, M., Nakahara, K. et al. Top-down signal from prefrontal cortex in executive control of memory retrieval. Nature 401, 699–703 (1999). https://doi.org/10.1038/44372
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/44372
This article is cited by
-
Regulation of social interaction in mice by a frontostriatal circuit modulated by established hierarchical relationships
Nature Communications (2023)
-
Brain-wide mapping reveals that engrams for a single memory are distributed across multiple brain regions
Nature Communications (2022)
-
Mental construction of object symbols from meaningless elements by Japanese macaques (Macaca fuscata)
Scientific Reports (2022)
-
Prefrontal feature representations drive memory recall
Nature (2022)
-
Disrupting self-evaluative processing with electrostimulation mapping during awake brain surgery
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.