Abstract
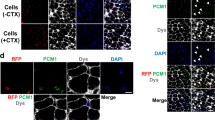
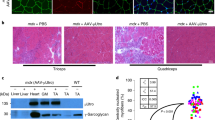
The development of cell or gene therapies for diseases involving cells that are widely distributed throughout the body has been severely hampered by the inability to achieve the disseminated delivery of cells or genes to the affected tissues or organ1. Here we report the results of bone marrow transplantation studies in the mdx mouse, an animal model of Duchenne's muscular dystrophy2, which indicate that the intravenous injection of either normal haematopoietic stem cells or a novel population of muscle-derived stem cells into irradiated animals results in the reconstitution of the haematopoietic compartment of the transplanted recipients, the incorporation of donor-derived nuclei into muscle, and the partial restoration of dystrophin expression in the affected muscle. These results suggest that the transplantation of different stem cell populations, using the procedures of bone marrow transplantation, might provide an unanticipated avenue for treating muscular dystrophy as well as other diseases where the systemic delivery of therapeutic cells to sites throughout the body is critical. Our studies also suggest that the inherent developmental potential of stem cells isolated from diverse tissues or organs may be more similar than previously anticipated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mulligan,R. C. The basic science of gene therapy. Science 260, 926–932 (1993).
Sicinski,P. et al. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science 244, 1578–1580 (1989).
Hall,Z. W. & Ralston,E. A. Nuclear domains in muscle cells. Cell 59, 771–772 (1989).
Karpati,G. et al. Dystrophin is expressed in mdx skeletal muscle fibers after normal myoblast implantation. Am. J. Pathol. 135, 27–32 (1989).
Pavlath,G. K., Rich,K., Webster,S. G. & Blau,H. M. Localization of muscle gene products in nuclear domains. Nature 337, 570–573 (1989).
Gussoni,E., Blau,H. M. & Kunkel,L. M. The fate of individual myoblasts after transplantation into muscles of DMD patients. Nature Med. 3, 970–977 (1997).
Hoffman,E. P., Morgan,J. E., Watkins,S. C. & Partridge,T. A. Somatic reversion/suppression of the mouse mdx phenotype in vivo. J. Neurol. Sci. 99, 9–25 (1990).
Ferrari,G. et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science 279, 1528–1530 (1998).
Bittner,R. E. et al. Recruitment of bone marrow-derived cells by skeletal and cardiac muscle in adult dystrophic mdx mice. Anat. Embryol. (Berl.) 199, 391–396 (1999).
Goodell,M. A., Brose,K., Paradis,G., Conner,A. S. & Mulligan,R. C. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J. Exp. Med. 183, 1797–1806 (1996).
Goodell,M. A. et al. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nature Med. 3, 1337–1345 (1997).
Rando,T. A. & Blau,H. M. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J. Cell Biol. 125, 1275–1287 (1994).
Mauro,A. Satellite cells of skeletal muscle. J. Biophys. Biochem. Cytol. 9, 493–495 (1961).
Partridge,T. A., Morgan,J. E., Coulton,G. R., Hoffman,E. P. & Kunkel,L. M. Conversion of mdx myofibers from dystrophin negative to positive by injection of normal myoblasts. Nature 337, 176–179 (1989).
Partridge,T. A. Invited review: myoblast transfer: a possible therapy for inherited myopathies? Muscle Nerve 14, 197–212 (1991).
Acsadi,G. et al. Human dystrophin expression in mdx mice after intramuscular injections of DNA constructs. Nature 352, 815–818 (1991).
Acsadi,G. et al. Human dystrophin expression in mdx mice after intramuscular injections of DNA constructs. Nature 352, 815–818 (1991).
Ragot,T. et al. Efficient adenovirus-mediated transfer of a human minidystrophin gene to skeletal muscle of mdx mice. Nature 361, 647–650 (1993).
Kochanek,S. et al. A new adenoviral vector: replacement of all viral coding sequences with 28 kb of DNA independently expressing both full-length dystrophin and b-galactosidase. Proc. Natl Acad. Sci. USA 93, 5731–5736 (1996).
Rafael,J. A. et al. Prevention of dystrophic pathology in mdx mice by a truncated dystrophin isoform. Hum. Mol. Genet. 3, 1725–1733 (1994).
Phelps,S. F. et al. Expression of full-length and truncated dystrophin mini-genes in transgenic mdx mice. Hum. Mol. Genet. 4, 1251–1258 (1995).
Pereira,R. F. et al. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc. Natl Acad. Sci. USA 92, 4857–4861 (1995).
Saito,T. et al. Myogenic expression of mesenchymal stem cells within myotubes of mdx mice in vitro and in vivo. Tissue Eng. 1, 327–343 (1995).
Prockop,D. J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276, 71–74 (1997).
Asahara,T. et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–967 (1997).
Shi,Q. et al. Evidence for circulating bone marrow-derived endothelial cells. Blood 92, 362–367 (1998).
Bjornson,C. R., Rietze,R. L., Reynolds,B. A., Magli,M. C. & Vescovi,A. L. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science 283, 534–537 (1999).
Baroffio,A., Bochaton-Piallat, M.-L., Gabbiani,G. & Bader,C. R. Heterogeneity in the progeny of single human muscle satellite cells. Differentiation 59, 259–268 (1995).
Lichter,P. et al. Rapid detection of human chromosome 21 aberrations by in situ hybridization. Proc. Natl Acad. Sci. USA 85, 9664–9668 (1988).
Gussoni,E. et al. A method to codetect introduced genes and their products in gene therapy protocols. Nature Biotech. 14, 1012–1016 (1996).
Acknowledgements
We thank E. Snyder for the mouse Y-chromosome probe. This work was supported by the Muscular Dystrophy Association (L.M.K.), the Bernard and Alva Gimbel Foundation and Family (L.M.K.), the Howard Hughes Medical Institute (L.M.K., R.C.M.) and the NIH (R.C.M.). L.M.K. and R.C.M. are Investigators of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Gussoni, E., Soneoka, Y., Strickland, C. et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 401, 390–394 (1999). https://doi.org/10.1038/43919
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/43919
This article is cited by
-
MuSCs and IPCs: roles in skeletal muscle homeostasis, aging and injury
Cellular and Molecular Life Sciences (2024)
-
Long-term longitudinal study on swine VML model
Biology Direct (2023)
-
Intermuscular adipose tissue in metabolic disease
Nature Reviews Endocrinology (2023)
-
Human MuStem cells repress T-cell proliferation and cytotoxicity through both paracrine and contact-dependent pathways
Stem Cell Research & Therapy (2022)
-
Functional Neuronal Differentiation of Injury-Induced Muscle-Derived Stem Cell-Like Cells with Therapeutic Implications
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.