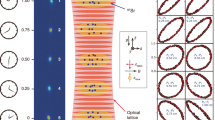
Abstract
One of the most striking predictions of Einstein's special theory of relativity is also perhaps the best known formula in all of science: E=mc2. If this equation were found to be even slightly incorrect, the impact would be enormous — given the degree to which special relativity is woven into the theoretical fabric of modern physics and into everyday applications such as global positioning systems. Here we test this mass–energy relationship directly by combining very accurate measurements of atomic-mass difference, Δm, and of γ-ray wavelengths to determine E, the nuclear binding energy, for isotopes of silicon and sulphur. Einstein's relationship is separately confirmed in two tests, which yield a combined result of 1−Δmc2/E=(−1.4±4.4)×10−7, indicating that it holds to a level of at least 0.00004%. To our knowledge, this is the most precise direct test of the famous equation yet described.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Mohr, P. J. & Taylor, B. N. Rev. Mod. Phys. 77, 1–107 (2005).
Kessler, E. G. et al. Phys. Lett. A 255, 221–229 (1999).
Kessler, E. G. et al. Nucl. Instrum. Meth. Phys. Res. A 457, 187–202 (2001).
Dewey, M. S. et al. Preprint nucl-ex/0507011 at http://arxiv.org (2005).
Rainville, S., Thompson, J. K. & Pritchard, D. E. Science 303, 334–338 (2004).
Audi, G., Wapstra, A. H. & Thibault, C. Nucl. Phys. A 729, 337–676 (2003).
Thompson, J. K., Rainville, S. & Pritchard, D. E. Nature 430, 58–61 (2004).
NIST Chemistry WebBook, NIST Standard Reference Database Number 69 (eds Linstrom, P. J. & Mallard, W. G.) (National Institute of Standards and Technology, Gaithersburg, Maryland, 2003).
Chase, M. W. J. Phys. Chem. Ref. Data Monogr. 9, 1–1951 (1998).
Greene, G. L., Dewey, M. S., Kessler, E. G. & Fischbach, E. Phys. Rev. D 44, R2216–R2219 (1991).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
(DOC 59 kb)
Rights and permissions
About this article
Cite this article
Rainville, S., Thompson, J., Myers, E. et al. A direct test of E=mc2. Nature 438, 1096–1097 (2005). https://doi.org/10.1038/4381096a
Published:
Issue Date:
DOI: https://doi.org/10.1038/4381096a
This article is cited by
-
The fundamental constants of physics and the International System of Units
Rendiconti Lincei. Scienze Fisiche e Naturali (2021)
-
What Do We Mean by “True” in Scientific Realism?
Foundations of Science (2020)
-
The Measurement of the Silicon Lattice Parameter and the Count of Atoms to Realise the Kilogram
MAPAN (2020)
-
Quantum formulation of the Einstein equivalence principle
Nature Physics (2018)
-
Balancing energy and mass with neutrons
Nature Physics (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.