Packaging red-rose anthocyanin as part of a ‘superpigment’ in another flower turns it brilliant blue.
Abstract
The same anthocyanin pigment makes roses red but cornflowers blue1, a phenomenon that has so far not been entirely explained. Here we describe the X-ray crystal structure of the cornflower pigment, which reveals that its blue colour arises from a complex of six molecules each of anthocyanin and flavone, with one ferric iron, one magnesium and two calcium ions. We believe that this tetrametal complex may represent a previously undiscovered type of supermolecular pigment.
Similar content being viewed by others
Main
An anthocyanin pigment was first isolated in 1913 as a red oxonium salt from the blue cornflower Centaurea cyanus2, and later the same pigment was discovered in a red rose3. The colour variation was variously ascribed to a difference in flower-petal pH (ref. 3), an association of the pigment with metal ions4 or with another pigment5. However, the blue-cornflower pigment (later named protocyanin6,7) was found to contain iron and magnesium8,9 in complex with anthocyanin and flavone10,11; calcium is also essential for the reconstruction of protocyanin12.
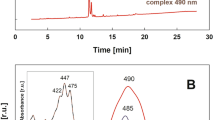
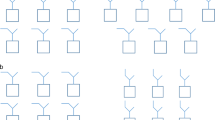
We determined the crystal structure of reconstructed protocyanin at 1.05 Å resolution. (For methods, see supplementary information.) In the refined molecule, the four metal ions Fe3+, Mg2+ and two Ca2+ align along the pseudo-three-fold axis (Fig. 1a) and are coordinated to six anthocyanin (cyanidin 3-O-(6-O-succinyl glucoside)-5-O-glucoside) and six flavone (apigenin 7-O-glucuronide-4′-O-(6-O-malonyl-glucoside)) molecules. The inner Fe3+ and Mg2+ ions are each coordinated to three anthocyanin molecules, respectively, and the two outer Ca2+ ions are each coordinated to three flavone molecules (see supplementary information).
a, Side view along the pseudo-three-fold axis. Blue, anthocyanin; yellow, flavone glycoside; spheres: red, Fe3+; green, Mg2+; black, Ca2+. For stereo view, see supplementary information. b, Side views of stacking pigment pairs. Left, one anthocyanin molecule binds Fe3+ (red), the other binds Mg2+ (green); centre, two flavones each bind to one Ca2+ (black); right, flavone and anthocyanin molecules bind to Ca2+ and Mg2+, respectively.
The two sites of the central nuclei have the same electron density, which corresponds to the average for Fe3+ and Mg2+ (results not shown). This suggests that the sites are occupied by either Fe3+ or Mg2+ because of the random orientation of the molecule along the pseudo-three-fold axis. To verify this, we investigated the X-ray structures of metal-substituted FeMgBa-, FeMnBa- and FeCdBa-protocyanins (results not shown). (Barium derivatives are more stable than calcium derivatives for isolation as crystals.)

In the FeMgBa-protocyanin, the electron density of the inner nuclei is almost the same as in protocyanin, whereas in the FeMnBa- and FeCdBa-protocyanins it is the average of Fe3+ and of the substituted ions, respectively. The distances between the two metals and the coordinating oxygen atoms vary according to the radii of the substituted metals. These results indicate that the two metals are heterogeneous. We conclude that the inner nuclei of protocyanin consist of Fe3+ and Mg2+ ions.
In the protocyanin molecule, the anthocyanins and flavones self-associate in pairs. The Fe3+ and Mg2+ ions are coordinated to different anthocyanin fragments in an anthocyanin pair (Fig. 1b, left); the two Ca2+ ions are coordinated with separate flavone fragments in a flavone pair (Fig. 1b, centre). Stacking of anthocyanin and flavone (co-pigmentation) is also evident (Fig. 1b, right). The C–C and C–O bond lengths in the B-ring of anthocyanin (data not shown) indicate that the anthocyanin is in the 4′-keto-quinoidal form.
Blue flower colours are caused mainly by delphinidin-type anthocyanins — for example, commelinin13. In protocyanin, however, the blue colour is produced by a cyanidin-type anthocyanin. The chelation of Fe3+ and Mg2+ with the 4′-keto-quinoidal base of anthocyanin is important for the blueing in protocyanin. The two Ca2+ ions coordinate with the flavones to form components that bring about co-pigmentation as well as stabilization of the molecule. The blue colour in protocyanin is therefore developed by a tetranuclear metal complex, which may represent a new type of supermolecular pigment.
References
Brouillard, R. & Dangles, O. in The Flavonoids, Advances in Research since 1986 (ed. Harborne, J. B.) 565–588 (Chapman and Hall, London, 1994).
Willstätter, R. & Everest, R. W. Justus Liebigs Ann. Chem. 401, 189–232 (1913).
Willstätter, R. & Mallison, H. Justus Liebigs Ann. Chem. 408, 147–162 (1915).
Shibata, K., Shibata, Y. & Kashiwagi, I. J. Am. Chem. Soc. 41, 208–220 (1919).
Robinson, G. M. & Robinson, R. Biochem. J. 25, 1687–1705 (1931).
Bayer, E. Chem. Ber. 91, 1115–1122 (1958).
Bayer, E., Egeter, H., Fink, A., Nether, K. & Wegmann, K. Angew. Chem. 78, 834–841 (1966).
Hayashi, K., Saito, N. & Mitsui, S. Proc. Jpn Acad. 37, 393–397 (1961).
Saito, N., Mitsui, S. & Hayashi, K. Proc. Jpn Acad. 37, 485–490 (1961).
Kondo, T. et al. Angew. Chem. Int. Ed. Engl. 33, 978–979 (1994).
Kondo, T., Ueda, M., Isobe, M. & Goto, T. Tetrahedron Lett. 39, 8307–8310 (1998).
Takeda, K. et al. Phytochemistry 66, 1607–1613 (2005).
Kondo, T. et al. Nature 358, 515–518 (1992).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary methods (MS word 36KB)
(DOC 34 kb)
Supplementary figure (MS word 1.0 MB)
(DOC 1003 kb)
Rights and permissions
About this article
Cite this article
Shiono, M., Matsugaki, N. & Takeda, K. Structure of the blue cornflower pigment. Nature 436, 791 (2005). https://doi.org/10.1038/436791a
Published:
Issue Date:
DOI: https://doi.org/10.1038/436791a
This article is cited by
-
Assessment of violet-blue color formation in Phalaenopsis orchids
BMC Plant Biology (2020)
-
The impact of cultivation systems on the nutritional and phytochemical content, and microbiological contamination of highbush blueberry
Scientific Reports (2020)
-
Disorder in convergent floral nanostructures enhances signalling to bees
Nature (2017)
-
On the structure of Zn(II) and Cu(II) cyanin complexes in aqueous solution
Structural Chemistry (2014)
-
Anatomical and biochemical studies of bicolored flower development in Muscari latifolium
Protoplasma (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.