Abstract
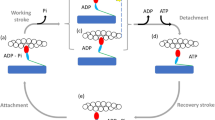
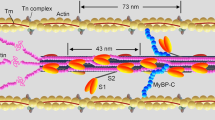
Muscles generate force and shortening in a cyclical interaction between the myosin head domains projecting from the myosin filaments and the adjacent actin filaments. Although many features of the dynamic performance of muscle are determined by the rates of attachment and detachment of myosin and actin1, the primary event in force generation is thought to be a conformational change or ‘working stroke’ in the actin-bound myosin head2,3,4,5,6,7,8. According to this hypothesis, the working stroke is much faster than attachment or detachment, but can be observed directly in the rapid force transients that follow step displacement of the filaments3. Although many studies of the mechanism of muscle contraction9,10,11,12,13 have been based on this hypothesis, the alternative view—that the fast force transients are caused by fast components of attachment and detachment14,15,16,17 —has not been excluded definitively. Here we show that measurements of the axial motions of the myosin heads at ångström resolution by a new X-ray interference technique18 rule out the rapid attachment/detachment hypothesis, and provide compelling support for the working stroke model of force generation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Huxley, A. F. Muscle structure and theories of contraction. Prog. Biophys. Biophys. Chem. 7, 255–318 (1957).
Huxley, H. E. The mechanism of muscle contraction. Science 164, 1356–1366 (1969).
Huxley, A. F. & Simmons, R. M. Proposed mechanism of force generation in striated muscle. Nature 233, 533–538 (1971).
Huxley, A. F. Muscular contraction (review lecture). J. Physiol. (Lond.) 243, 1–43 (1974).
Rayment, I. et al. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science 261, 50–58 (1993).
Rayment, I. et al. Structure of the actin–myosin complex and its implications for muscle contraction. Science 261, 58–65 (1993).
Dominguez, R., Freyzon, Y., Trybus, K. M. & Cohen, C. Crystal structure of a vertebrate smooth muscle myosin motor domain and its complex with the essential light chain: visualisation of the pre-power stroke state. Cell 94, 559–571 (1998).
Geeves, M. A. & Holmes, K. C. Structural mechanism of muscle contraction. Annu. Rev. Biochem. 68, 687–728 (1999).
Huxley, H. E. et al. Changes in the X-ray reflections from contracting muscle during rapid mechanical transients and their structural implications. J. Mol. Biol. 169, 469–506 (1983).
Irving, M., Lombardi, V., Piazzesi, G. & Ferenczi, M. A. Myosin head movements are synchronous with the elementary force-generating process in muscle. Nature 357, 156–158 (1992).
Lombardi, V., Piazzesi, G. & Linari, M. Rapid regeneration of the actin–myosin power stroke in contracting muscle. Nature 355, 638–641 (1992).
Irving, M. et al. Conformation of the myosin motor during force generation in skeletal muscle. Nature Struct. Biol. 7, 482–485 (2000).
Corrie, J. E. T. et al. Dynamic measurement of myosin light-chain domain tilt and twist in muscle contraction. Nature 400, 425–430 (1999).
Podolsky, R. J. & Nolan, A. C. Muscle contraction transients, cross-bridge kinetics, and the Fenn effect. Cold Spring Harb. Symp. Quant. Biol. 37, 661–668 (1973).
Brenner, B. Rapid dissociation and reassociation of actomyosin cross-bridges during force generation: a newly observed facet of cross-bridge action in muscle. Proc. Natl Acad. Sci. USA 88, 10490–10494 (1991).
Kitamura, K., Tokunaga, M., Iwane, A. H. & Yanagida, T. A single myosin head moves along an actin filament with regular steps of 5.3 nanometres. Nature 397, 129–134 (1999).
Howard, J. Mechanics of Motor Proteins and the Cytoskeleton (Sinauer, Sunderland, MA, 2001).
Linari, M. et al. Interference fine structure and sarcomere length dependence of the axial X-ray pattern from active single muscle fibres. Proc. Natl Acad. Sci. USA 97, 7226–7231 (2000).
Ford, L. E., Huxley, A. F. & Simmons, R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J. Physiol. (Lond.) 269, 441–515 (1977).
Dobbie, I. et al. Elastic bending and active tilting of myosin heads during muscle contraction. Nature 396, 383–387 (1998).
Linari, M. et al. The stiffness of skeletal muscle in isometric contraction and rigor: the fraction of myosin heads bound to actin. Biophys. J. 74, 2459–2473 (1998).
Piazzesi, G. et al. Changes in conformation of myosin heads during the development of isometric contraction and rapid shortening in single frog muscle fibres. J. Physiol. (Lond.) 514, 305–312 (1999).
Lombardi, V. & Piazzesi, G. The contractile response during steady lengthening of stimulated frog muscle fibres. J. Physiol. (Lond.) 431, 141–171 (1990).
Huxley, A. F., Lombardi, V. & Peachey, L. D. A system for fast recording of longitudinal displacement of a striated muscle fibre. J. Physiol. (Lond.) 317, 12P–13P (1981).
Boesecke, P., Diat, O. & Rasmussen, B. High-brilliance beamline at the European Synchrotron Radiation Facility. Rev. Sci. Instrum. 66, 1636–1638 (1995).
Haselgrove, J. C. X-ray evidence for conformational changes in the myosin filaments of vertebrate striated muscle. J. Mol. Biol. 92, 113–143 (1975).
Sjöström M. & Squire, J. M. Fine structure of the A band in cryosections: the structure of the A-band of human skeletal muscle fibres from ultrathin cryosections negatively stained. J. Mol. Biol. 109, 49–68 (1977).
Craig, R. Structure of A-segments from frog and rabbit skeletal muscle. J. Mol. Biol. 109, 69–81 (1977).
Thorson, J. W. & White, D. C. S. Distributed representations for actin-myosin interaction in the oscillatory contraction of muscle. Biophys. J. 9, 360–390 (1969).
Ford, L. E., Huxley, A. F. & Simmons, R. M. The relation between stiffness and filament overlap in stimulated frog muscle fibres. J. Physiol. (Lond) 311, 219–249 (1981).
Acknowledgements
This work was supported by the Medical Research Council, Consiglio Nazionale della Ricerche (CNR), Ministero dell'Istruzione, dell'Università e della Ricerca (MURST), Telethon (Italy), EMBL, EU and ESRF. We thank A. F. Huxley for comments, J. Gorini, A. Aiazzi and M. Dolfi for mechanical and electronics support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Piazzesi, G., Reconditi, M., Linari, M. et al. Mechanism of force generation by myosin heads in skeletal muscle. Nature 415, 659–662 (2002). https://doi.org/10.1038/415659a
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/415659a
This article is cited by
-
Monitoring the myosin crossbridge cycle in contracting muscle: steps towards ‘Muscle—the Movie’
Journal of Muscle Research and Cell Motility (2019)
-
Synchrotron radiation X-ray diffraction studies on muscle: past, present, and future
Biophysical Reviews (2019)
-
The Sticking Point in the Bench Press, the Squat, and the Deadlift: Similarities and Differences, and Their Significance for Research and Practice
Sports Medicine (2017)
-
Harmonic force spectroscopy measures load-dependent kinetics of individual human β-cardiac myosin molecules
Nature Communications (2015)
-
The influence of filament elasticity on transients after sudden alteration of length of muscle or load
European Biophysics Journal (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.