Abstract
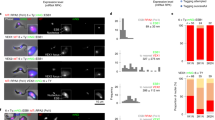
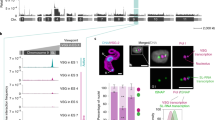
In the mammalian host, African trypanosomes generate consecutive waves of parasitaemia by changing their antigenic coat. Because this coat consists of a single type of variant surface glycoprotein (VSG), the question arises of how a trypanosome accomplishes the transcription of only one of a multi-allelic family of VSG expression site loci to display a single VSG type on the surface at any one time1. No major differences have been detected between the single active expression site and the cohort of inactive expression sites2. Here we identify an extranucleolar body containing RNA polymerase I (pol I) that is transcriptionally active and present only in the bloodstream form of the parasite. Visualization of the active expression site locus by tagging with green fluorescent protein3 shows that it is specifically located at this unique pol I transcriptional factory. The presence of this transcriptional body in postmitotic nuclei and its stability in the nucleus after DNA digestion provide evidence for a coherent structure. We propose that the recruitment of a single expression site and the concomitant exclusion of inactive loci from a discrete transcriptional body define the mechanism responsible for VSG mono-allelic expression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others

References
Cross, G. A. M. Identification, purification and properties of variant-specific glycoprotein antigens constituting the surface coat of Trypanosoma brucei. Parasitology 71, 393–417 (1975).
Borst, P. & Ulbert, S. Control of VSG gene expression sites. Mol. Biochem. Parasitol. 114, 17–27 (2001).
Robinett, C. C. et al. In vivo localization of DNA sequences and visualization of large-scale chromatin organization using lac operator/repressor recognition. J. Cell Biol. 135, 1685–700 (1996).
Pays, E., Lips, S., Nolan, D., Vanhamme, L. & Perez-Morga, D. The VSG expression sites of Trypanosoma brucei: multipurpose tools for the adaptation of the parasite to mammalian hosts. Mol. Biochem. Parasitol. 114, 1–16 (2001).
Bitter, W., Gerrits, H., Kieft, R. & Borst, P. The role of transferrin-receptor variation in the host range of Trypanosoma brucei. Nature 391, 499–502 (1998).
Pays, E. et al. The genes and transcripts of an antigen expression site from T. brucei. Cell 57, 835–845 (1989).
Zomerdijk, J. C. B. M. et al. The promoter for a variant surface glycoprotein gene expression site in Trypanosoma brucei. EMBO J. 9, 2791–2801 (1990).
Borst, P. & Chaves, I. Mono-allelic expression of genes in simple eukaryotes. Trends Genet. 15, 95–96 (1999).
Robinson, N. P., Burman, N., Melville, S. E. & Barry, J. D. Predominance of duplicative VSG gene conversion in antigenic variation in African trypanosomes. Mol. Cell. Biol. 19, 5839–5846 (1999).
Roditi, I. et al. Procyclin gene expression and loss of the variant surface glycoprotein during differentiation of Trypanosoma brucei. J. Cell Biol. 108, 737–746 (1989).
Kooter, J. M. & Borst, P. Alpha-amanitin-insensitive transcription of variant surface glycoprotein genes provides further evidence for discontinuous transcription in trypanosomes. Nucleic Acids Res. 12, 9457–9472 (1984).
Rudenko, G., Bishop, D., Gottesdiener, K. & Van der Ploeg, L. H. T. Alpha-amanitin resistant transcription of protein coding genes in insect and bloodstream form Trypanosoma brucei. EMBO J. 8, 4259–4263 (1989).
Rudenko, G., Lee, M. G.-S. & Van der Ploeg, L. H. T. The PARP and VSG genes of Trypanosoma brucei do not resemble RNA polymerase II transcription units in sensitivity to Sarkosyl in nuclear run-on assays. Nucleic Acids Res. 20, 303–306 (1992).
Laufer, G., Schaaf, G., Bollgonn, S. & Günzl, A. In vitro analysis of alpha-amanitin-resistant transcription from the rRNA, procyclic acidic repetitive protein, and variant surface glycoprotein gene promoters in Trypanosoma brucei. Mol. Cell. Biol. 19, 5466–5473 (1999).
Scheer, U. & Hock, R. Structure and function of the nucleolus. Curr. Opin. Cell Biol. 11, 385–390 (1999).
Hartshorne, T. & Agabian, N. RNA B is the major nucleolar trimethylguanosine-capped small nuclear RNA associated with fibrillarin and pre-rRNAs in Trypanosoma brucei. Mol. Cell. Biol. 13, 144–154 (1993).
Pombo, A. et al. Regional specialization in human nuclei: visualization of discrete sites of transcription by RNA polymerase III. EMBO J. 18, 2241–2253 (1999).
Zomerdijk, J. C. B. M., Kieft, R. & Borst, P. Efficient production of functional mRNA mediated by RNA polymerase I in Trypanosoma brucei. Nature 353, 772–775 (1991).
Chaves, I. et al. Subnuclear localization of the active variant surface glycoprotein gene expression site in Trypanosoma brucei. Proc. Natl Acad. Sci. USA 95, 12328–12333 (1998).
Wirtz, E., Leal, S., Ochatt, C. & Cross, G. A. M. A tightly regulated inducible expression system for conditional gene knock-outs and dominant-negative genetics in Trypanosoma brucei. Mol. Biochem. Parasitol. 99, 89–101 (1999).
Chaves, I., Rudenko, G., Dirks-Mulder, A., Cross, M. & Borst, P. Control of variant surface glycoprotein gene-expression sites in Trypanosoma brucei. EMBO J. 18, 4846–4855 (1999).
Vanhamme, L. et al. Differential RNA elongation controls the variant surface glycoprotein gene expression sites of Trypanosoma brucei. Mol. Microbiol. 36, 328–340 (2000).
Navarro, M., Cross, G. A. & Wirtz, E. Trypanosoma brucei variant surface glycoprotein regulation involves coupled activation/inactivation and chromatin remodeling of expression sites. EMBO J. 18, 2265–2272 (1999).
Grosveld, F. Activation by locus control regions? Curr. Opin. Genet. Dev. 9, 152–157 (1999).
Cook, P. R. The organization of replication and transcription. Science 284, 1790–1795 (1999).
Ohlsson, R., Tycko, B. & Sapienza, C. Monoallelic expression: ‘there can only be one’. Trends Genet. 14, 435–438 (1998).
Matthews, K. R. & Gull, K. Evidence for an interplay between cell cycle progression and the initiation of differentiation between life cycle forms of African trypanosomes. J. Cell Biol. 125, 1147–1156 (1994).
Ersfeld, K. & Gull, K. Partitioning of large and minichromosomes in Trypanosoma brucei. Science 276, 611–614 (1997).
Navarro, M. & Cross, G. A. DNA rearrangements associated with multiple consecutive directed antigenic switches in Trypanosoma brucei. Mol. Cell. Biol. 16, 3615–3625 (1996).
Navarro, M. & Cross, G. A. In situ analysis of a variant surface glycoprotein expression-site promoter region in Trypanosoma brucei. Mol. Biochem. Parasitol. 94, 53–66 (1998).
Acknowledgements
We thank A. F. Straight for the GFP–LacI tagging constructs; E. Wirtz and C. Ochatt for pLew100 and the SAT-derived construct; M. Hoek and G. A. M. Cross for the BAC clone; G. Pierron for the fibrillarin (P2G3) monoclonal antibody; and K. E. Sawin for the anti-GFP rabbit polyclonal antibody. We are grateful to K. Ersfeld for FISH protocols, and D. Robinson for technical advice. We thank A. Baines for technical assistance at the initial stage of this work, and all members of the Gull laboratory for discussions. This work was founded by the Wellcome Trust and the BBSRC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
41586_2001_BF414759a_MOESM1_ESM.mov
Movie (MOV 1.2 MB)
Immunofluorescence analysis of the bloodstream form of T. brucei using an anti-pol-I antibody (red) and DAPI staining (blue). The three-dimensional nature of the pol I body in the nucleoplasm is shown.
(Click here to download the Quicktime plugin: http://www.apple.com/quicktime/download/ )
Rights and permissions
About this article
Cite this article
Navarro, M., Gull, K. A pol I transcriptional body associated with VSG mono-allelic expression in Trypanosoma brucei. Nature 414, 759–763 (2001). https://doi.org/10.1038/414759a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/414759a
This article is cited by
-
Physiology and pharmacological targeting of phase separation
Journal of Biomedical Science (2024)
-
Decoding the impact of nuclear organization on antigenic variation in parasites
Nature Microbiology (2023)
-
Genome-wide chromatin interaction map for Trypanosoma cruzi
Nature Microbiology (2023)
-
An allele-selective inter-chromosomal protein bridge supports monogenic antigen expression in the African trypanosome
Nature Communications (2023)
-
An assembly of nuclear bodies associates with the active VSG expression site in African trypanosomes
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.