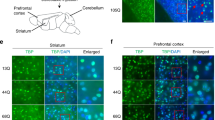
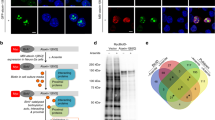
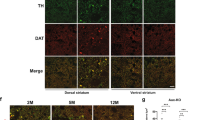
Abstract
Spinocerebellar ataxia type 1 (SCA1) is one of several neurodegenerative disorders caused by an expansion of a polyglutamine tract1,2. It is characterized by ataxia, progressive motor deterioration, and loss of cerebellar Purkinje cells1. To understand the pathogenesis of SCA1, we examined the subcellular localization of wild-type human ataxin-1 (the protein encoded by the SCA1 gene) and mutant ataxin-1 in the Purkinje cells of transgenic mice3. We found that ataxin-1 localizes to the nuclei of cerebellar Purkinje cells. Normal ataxin-1 localizes to several nuclear structures ∼0.5 µm across, whereas the expanded ataxin-1 localizes to a single ∼2-µm structure, before the onset of ataxia. Mutant ataxin-1 localizes to a single nuclear structure in affected neurons of SCA1 patients. Similarly, COS-1 cells transfected with wild-type or mutant ataxin-1 show a similar pattern of nuclear localization; with expanded ataxin-1 occurring in larger structures that are fewer in number than those of normal ataxin-1. Colocalization studies show that mutant ataxin-1 causes a specific redistribution of the nuclear matrix-associated domain containing promyelocytic leukaemia protein4,5,6,7. Nuclear matrix preparations demonstrate that ataxin-1 associates with the nuclear matrix in Purkinje and COS cells. We therefore propose that a critical aspect of SCA1 pathogenesis involves the disruption of a nuclear matrix-associated domain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
1. Zoghbi, H. Y. & Orr, H. T. Spinocerebellar ataxia type 1. Semin. Cell Biol. 6, 29–35 (1995).
2. Ross, C. A. When more is less: Pathogenesis of glutamine repeat neurodegenerative diseases. Neuron 15, 493–496 (1995).
3. Burright, E. N. et al. SCA1 transgenic mice: a model for neurodegeneration caused by an expanded CAG trinucleotide repeat. Cell 82, 937–948 (1995).
4. Stuurman, N. et al. Amonoclonal antibody recognizing nuclear matrix-associated nuclear bodies. J. Cell Sci. 101, 773–784 (1992).
5. Weis, K. et al. Retinoic acid regulates aberrant nuclear localization of PML-RAR alpha in acute promyelocytic leukemia cells. Cell 76, 345–356 (1994).
6. Koken, M. H. et al. The t(15;17) translocation alters a nuclear body in a retinoic acid-reversible fashion. EMBO J. 13, 1073–1083 (1994).
7. Dyck, J. A. et al. Anovel macromolecular structure is a target of the promyelocyte-retinoic acid receptor oncoprotein. Cell 76, 333–343 (1994).
8. Servadio, A. et al. Expression analysis of the ataxin-1 protein in tissues from normal and spinocerebellar ataxia type 1 individuals. Nature Genet. 10, 94–98 (1995).
9. Matilla, A. et al. Ataxin-1, the SCA1 gene product, is required for learning tasks mediated by both the hippocampus and cerebellum. Proc. Natl Acad. Sci. USA(submitted).
10. Robitaille, Y., Schut, L. & Kish, S. J. Structural and immunocytochemical features of olivopontocerebellar atrophy caused by the spinocerebellar ataxia type 1 (SCA-1) mutation define a unique phenotype. Acta Neropathol. 90, 572–581 (1995).
11. Spector, D. L. Macromolecular domains within the cell nucleus. Annu Rev. Cell Biol. 9, 265–315 (1993).
12. Fu, X. D. & Maniatis, T. Factor required for mammalian spliceosome assembly is localized to discrete regions in the nucleus. Nature 343, 437–441 (1990).
13. Raska, I. et al. Immunological and ultrastructural studies of the nuclear coiled body with autoimmune antibodies. Exp. Cell Res. 195, 27–37 (1991).
14. Dhordain, P. et al. The BTB/POZ domain targets the LAZ3/BCL6 oncoprotein to nuclear dots and mediates homomerisation in vivo. Oncogene 11, 2689–2697 (1995).
15. Bisotto, S., Lauriault, P., Duval, M. & Vincent, M. Colocalization of a high molecular mass phosphoprotein of the nuclear matrix (p255) with spliceosomes. J. Cell Sci. 108, 1873–1882 (1995).
16. Roizin, S. S. & Liu, J. C. Neuronal nuclear-cytoplasmic changes in Huntington's Chorea: electron microscope investigations. Adv. Neurol. 23, 95–122 (1979).
17. Tellez-Nagel, I., Johnson, A. B. & Terry, R. D. Studies on brain biopsies of patients with Huntington's chorea. J. Neuropathol. Exp. Neurol. 33, 308–332 (1974).
18. Roos, R. A. C. & Bots, G. T. A. M. Nuclear membrane indentations in Huntington's Chorea. J. Neurol. Sci. 61, 37–47 (1983).
19. Paulson, H. L. et al. Intranuclear inclusions of expanded polyglutamine protein in spinocerebellar ataxia type 3. Neuron 19, 333–344 (1997).
20. Davies, S. W. et al. Formation of neuronal intranuclear inclusions (NII) underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell 90, 537–548 (1997).
21. Matilla, A. et al. The cerebellar leucine-rich acidic nuclear protein interacts with ataxin-1. Nature 389, 974–978 (1997).
22. Cattoretti, G. et al. Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J. Pathol. 171, 83–98 (1993).
23. Banfi, S. et al. Identification and characterization of the gene causing type 1 spinocerebellar ataxia. Nature Genet 7, 513–520 (1994).
24. Ausubel, F. M. et al. in Current Protocols in Molecular Biology 9.2.1–9.2.3 (John Wiley, New York, (1996)).
25. Andrade, L. E., Tan, E. M. & Chan, E. K. Immunocytochemical analysis of the coiled body in the cell cycle and during cell proliferation. Proc. Natl Acad. Sci. USA 90, 1947–1951 (1993).
26. Chan, E. K., Takano, S., Andrade, L. E., Hamel, J. C. & Matera, A. G. Structure, expression and chromosomal localization of human p80-coilin gene. Nucleic Acids Res. 22, 4462–4469 (1994).
27. Tawfic, S. & Ahmed, K. Association of casein kinase 2 with nuclear matrix. Possible role in nuclear matrix protein phosphorylation. J. Biol. Chem. 69, 7489–7493 (1994).
28. Fey, E. G. & Penman, S. Nuclear matrix proteins reflect cell type origin in cultured human cells. Proc. Natl Acad. Sci. USA 85, 121–125 (1988).
29. Berezney, R. & Coffey, D. S. Nuclear matrix isolation and characterization of a framework structure from rat liver nuclei. J. Cell Biol. 73, 616–632 (1977).
Acknowledgements
We thank V. Bardwell for assistance with the BCL-6 transfections; E. N. Burright for assistance with SCA1 transgenic mice; D. Saxon for assistance with tissue sectioning and staining; G.Sedgewick for assistance with confocal microscopy and image processing; D. Armstrong for assistance in analysis of SCA1 human tissue; T. Maniatis for the anti-SC35 antibody; A. Dejean for the anti-PML polysera; R. Van Driel for the anti-PML monoclonal antibody 5E10; and E. K. Chan for the anti-p80-coilin polysera. This work was supported by grants from the National Institute of Neurological Disorders and Stroke of the NIH to H.T.O. and H.Y.Z. H.Y.Z. is a Howard Hughes Medical Institute investigator.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Skinner, P., Koshy, B., Cummings, C. et al. Ataxin-1 with an expanded glutamine tract alters nuclear matrix-associated structures. Nature 389, 971–974 (1997). https://doi.org/10.1038/40153
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/40153
This article is cited by
-
Intercellular Propagation and Aggregate Seeding of Mutant Ataxin-1
Journal of Molecular Neuroscience (2022)
-
Human Induced Pluripotent Stem Cell-Based Modelling of Spinocerebellar Ataxias
Stem Cell Reviews and Reports (2022)
-
Nucleophagy: from homeostasis to disease
Cell Death & Differentiation (2019)
-
Motor Performances of Spontaneous and Genetically Modified Mutants with Cerebellar Atrophy
The Cerebellum (2019)
-
Non-apoptotic cell death in animal development
Cell Death & Differentiation (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.