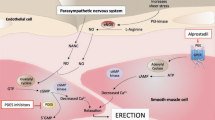
Abstract
Sildenafil citrate has been shown to be effective in a wide range of patients with erectile dysfunction and has been approved in the United States for this indication. The overall clinical safety of oral sildenafil, a potent inhibitor of phosphodiesterase type 5, in the treatment of erectile dysfunction was evaluated in more than 3700 patients (with a total of 1631 years of exposure worldwide). Safety and tolerability data were analysed from a series of double-blind, placebo-controlled studies and from 10 open-label extension studies of sildenafil in the treatment of erectile dysfunction. A total of 4274 patients (2722 sildenafil, 1552 placebo; age range 19–87 y) received double-blind treatment over a period of up to six months’ duration, and 2199 received long-term, open-label sildenafil for up to 1 y. The most commonly reported adverse events (all causes) were headache (16% sildenafil, 4% placebo), flushing (10% sildenafil, 1% placebo), and dyspepsia (7% sildenafil, 2% placebo) and they were predominantly transient and mild or moderate in nature. These adverse events reflect the pharmacology of sildenafil as a phosphodiesterase type 5 inhibitor. No cases of priapism were reported. The rate of discontinuation due to adverse events (all causes) was comparable for patients treated with sildenafil (2.5%) and placebo (2.3%). In open-label extension studies, 90% of patients completed long-term sildenafil treatment, with only 2% withdrawing due to adverse events. Sildenafil is a well-tolerated oral treatment for erectile dysfunction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Morales, A., Gingell, C., Collins, M. et al. Clinical safety of oral sildenafil citrate (VIAGRATM) in the treatment of erectile dysfunction. Int J Impot Res 10, 69–73 (1998). https://doi.org/10.1038/sj.ijir.3900354
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijir.3900354
Keywords
This article is cited by
-
Erektile Dysfunktion als Folge des Diabetes mellitus bei Männern
CME (2023)
-
Erektile Dysfunktion als Folge des Diabetes mellitus
Uro-News (2023)
-
Erektile Dysfunktion als Folge des Diabetes mellitus bei Männern
Info Diabetologie (2022)
-
Effects of phosphodiesterase type 5 inhibitors on choroid and ocular vasculature: a literature review
International Journal of Retina and Vitreous (2020)
-
Lipid-based nanoparticles in the treatment of erectile dysfunction
International Journal of Impotence Research (2020)