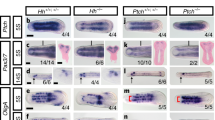
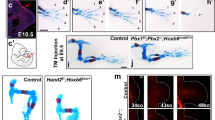
Abstract
Development of paired appendages at appropriate levels along the primary body axis is a hallmark of the body plan of jawed vertebrates. Hox genes are good candidates for encoding position in lateral plate mesoderm along the body axis 1,2 and thus for determining where limbs are formed. Local application of fibroblast growth factors (FGFs) to the anterior prospective flank of a chick embryo induces development of an ectopic wing, and FGF applied to posterior flank induces an ectopic leg3. If particular combinations of Hox gene expression determine where wings and legs develop, then formation of additional limbs from flank should involve changes in Hox gene expression that reflect the type of limb induced. Here we show that the same population of flank cells can be induced to form either a wing or a leg, and that induction of these ectopic limbs is accompanied by specific changes in expression of three Hox genes in lateral plate mesoderm. This then reproduces, in the flank, expression patterns found at normal limb levels. Hox gene expression is reprogrammed in lateral plate mesoderm, but is unaffected in paraxial mesoderm. Independent regulation of Hox gene expression in lateral plate mesoderm may have been a key step in the evolution of paired appendages.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Charite, J., de Graaff, W., Shen, S. & Deschamps, J. Ectopic expression of Hoxb-8 causes duplication of 'he ZPA in the forelimb and homeotic transformation of axial structures Cell 78, 589–601 1994).
Rancourt, D. E., Tsuzuki, T. & Capecchi, M. R. Genetic interaction between hoxb-5 and hoxb-6 is revealed by nonallelic noncomplementation. Genes. Dev. 9, 108–122 (1995).
Cohn, M. J., lzpisùa-Belmonte, J. C., Abud, H. Heath, J. K. & Tickle, C. Fibroblast growth factors induce additional limb development from the flank of chick embryos. Cell 80, 739–746 (1995).
Tickle, C. Vertebrate limb development. Curr. Biol. 5, 478–484 (1995).
Crossley, P. H., Minowada, G., MacArthur, C. A. & Martin, G. R. Roles for FGF8 in the induction, initiation, and maintenance of chick limb development. Cell 84, 127–136 (1996).
Akam, M. et al. The evolving role of Hox genes in arthropods. Development (suppl.) 209–215 (1994).
Lumsden, A. & Krumlauf, R. Patterning the vertebrate neuraxis. Science 274, 1109–1115 (1996).
Krumlauf, R. Hox genes in vertebrate development. Cell 78, 191–201 (1994).
Gibson-Brown, J. J. et al. Evidence of a role for T-Box genes in the evolution of limb morphogenesis and specification of forelimb-hindlimb identity. Mech. Dev. 56, 93–101 (1996).
Ohuchi, H. et al. An additional limb can be induced from the flank of the chick embryo by FGF4. Biochem. Biophys. Res. Commun. 209, 809–816 (1995).
Vogel, A., Rodriguez, C. & lzpisùa-Belmonte, J. C. Involvement of FGF-8 in initiation, outgrowth and patterning of the vertebrate limb. Development 122, 1737–1750 (1996).
Warren, R. W. Nagy, L., Selegue, J., Gates, J. & Carroll, S. Evolution of homeotic gene regulation and function in flies and butterflies. Nature 372, 458–461 (1994)
Simon, J. Locking in stable states of gene expression: transcriptional control during Drosophila development. Curr. Opin. Cell biol. 7, 376–385(1995).
Yu, B. D., Hess, J. L., Horning, S. E., & Brown, G. A. J. Korsmeyer, S. J. Altered Hox expression and segmental identity in Mll-mutant mice. Nature 378, 505–508 (1995).
Agar, W. E. The development of the anterior mesoderm, and paired fins with their nerves, in Lepidosiren and Protopterus. Trans. R. Soc. Edin. 45, 611–640 (1907).
Goodrich, E. S. Studies on the Structure and Development of Vertebrates. (Macmillan, London, 1930).
Thorogood, P. & Ferretti, P. Hox genes, fin folds and symmetry. Nature 364, 196 (1993).
Nieto, M. A., Patel, K. & Wilkinson, D. G. in Methods in Avian Embryology 220–235 (ed. Bronner-Fraser, M.) (Academic, San Diego, 1996).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cohn, M., Patel, K., Krumlauf, R. et al. Hox9 genes and vertebrate limb specification. Nature 387, 97–101 (1997). https://doi.org/10.1038/387097a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/387097a0
This article is cited by
-
The phenotypic morphology of human lumbar plexus roots associated with changes in the thoracolumbar vertebral count and trade-off
Scientific Reports (2020)
-
Contribution of neural crest-derived stem cells and nasal chondrocytes to articular cartilage regeneration
Cellular and Molecular Life Sciences (2020)
-
Incomplete duplication of a lower extremity (polymelia): a case report
Journal of Medical Case Reports (2014)
-
Conservation of linkage and evolution of developmental function within the Tbx2/3/4/5 subfamily of T-box genes: implications for the origin of vertebrate limbs
Development Genes and Evolution (2008)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.