Abstract
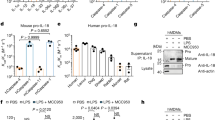
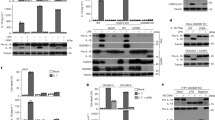
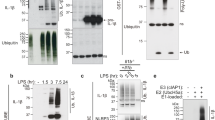
Interferon-γ-inducing factor (IGIF, interleukin-18) is a recently described cytokine that shares structural features with the inter-leukin-1 (IL-1) family of proteins and functional properties with IL-121–4. Like IL-12, IGIF is a potent inducer of interferon (IFN)-γ from T cells and natural killer cells1–3,5,6. IGIF is synthesized as a biologically inactive precursor molecule (proIGIF). The cellular production of IL-lβ, a cytokine implicated in a variety of inflammatory diseases, requires cleavage of its precursor (proIL-lβ) at an Asp-X site by interleukin-lβ-converting enzyme7,8 (ICE, recently termed caspase-19). The Asp-X sequence at the putative processing site in proIGIF2,3 suggests that a protease such as caspase-1 might be involved in the maturation of IGIF4. Here we demonstrate that caspase-1 processes proIGIF and proIL-lβ with equivalent efficiencies in vitro. A selective caspase-1 inhibitor blocks both lipopolysaccharide-induced IL-1β and IFN-γ production from human mononuclear cells. Furthermore, caspase-1-deficient mice are defective in lipopolysaccharide-induced IFN-γ production. Our results thus implicate caspase-1 in the physiological production of IGIF and demonstrate that it plays a critical role in the regulation of multiple proinflammatory cytokines. Specific caspase-1 inhibitors would provide a new class of anti-inflammatory drugs with multipotent action.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Nakamura, K. et al. Purification of a factor which provides a costimulatory signal for gamma interferon production. Infect. Immun. 61, 64–70 (1993).
Okamura, H. et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 378, 88–91 (1995).
Ushio, S. et al. Cloning of the cDNA for human IFN-gamma-inducing factor, expression in Escherichia coli, and studies on the biologic activities of the protein. J. Immunol. 156, 4274–4279 (1996).
Bazan, J. F., Timans, J. C. & Kastelein, R. A. A newly defined interleukin-1? Nature 379, 591 (1996).
Okamura, H. et al. A novel costimulatory factor for gamma interferon induction found in the livers of mice causes endotoxic shock. Infect. Immun. 63, 3966–3972 (1995).
Micallef, M. J. et al. Interferon-gamma-inducing factor enhances T helper 1 cytokine production by stimulated human T cells: synergism with interleukin-12 for interferon-gamma production. Eur. J. Immunol. 26, 1647–1651 (1996).
Thornberry, N. A. et al. A novel heterodimeric cysteine protease is required for interleukin- 1 beta processing in monocytes. Nature 356, 768–774 (1992).
Fraser, A. & Evan, G. A license to kill. Cell 85, 781–784 (1996).
Alnemri, E. S. et al. Human caspase-l/CED-3 protease nomenclature. Cell 87, 171 (1996).
Li, P. et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell 80, 401–411 (1995).
Kuida, K. et al. Altered cytokine export and apoptosis in mice deficient in interleukin- Ibeta converting enzyme. Science 267, 2000–2003 (1995).
Martin, S. J. & Green, D. R. Protease activation during apoptosis: death by a thousand cuts? Cell 82, 349–352 (1995).
Patel, T., Gores, G. J. & Kaufmann, S. H. The role of proteases during apoptosis. FASEB J. 10, 587–597 (1996).
Kuida, K. et al. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature 384, 368–372 (1996).
Kamens, J. et al. Identification and characterization of ICH-2, a novel member of the interleukin-1 beta-converting enzyme family of cysteine proteases. J. Biol. Chem. 270, 15250–15256 (1995).
Magram, J. et al. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity 4, 471–481 (1996).
Hunter, C. A., Chizzonite, R. & Remington, J. S. IL-lβ is required for IL-12 to induce production of IFN-γ by NK cells: a role for IL-lβ in the T cell independent mechanism of resistance against intracellular pathogens. J. Immunol. 155, 4347–4354 (1995).
D'Andrea, A. et al. Interleukin 10 (IL-10) inhibits human lymphocyte interferon-γ production by suppressing natural killer cell factor/IL-12 synthesis in accessory cells. J. Exp. Med. 178, 1041–1048 (1993).
Abbas, A. K., Murphy, K. M. & Sher, A. Functional diversity of helper T lymphocytes. Nature 383, 787–793 (1996).
Neurath, M. F. et al. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J. Exp. Med. 182, 1281–1290 (1995).
Campbell, I. L. et al. Essential role for interferon-gamma and interleukin-6 in autoimmune insulin-dependent diabetes in NOD/Wehi mice. J. Clin. Invest. 87, 739–742 (1991).
Locksley, R. M. Interleukin 12 in host defense against microbial pathogens. Proc. Natl Acad. Sci. USA 90, 5879–5880 (1993).
Gu, Y. et al. Activation of interferon-γ inducing factor mediated by interleukin-1β converting enzyme. Science 275, 206–209 (1997).
Fletcher, D. S. et al. A synthetic inhibitor of interleukin-1 beta converting enzyme prevents endotoxin-induced interleukin-1 beta production in vitro and in vivo. J. Interferon Cytokine Res. 15, 243–248 (1995).
Hugunin, M. et al. Protease activity of in vitro transcribed and translated Caenorhabditis elegans cell death gene (ced-3) product. J. Biol. Chem. 271, 3517–3522 (1996).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ghayur, T., Banerjee, S., Hugunin, M. et al. Caspase-1 processes IFN-γ-inducing factor and regulates LPS-induced IFN- γ production. Nature 386, 619–623 (1997). https://doi.org/10.1038/386619a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/386619a0
This article is cited by
-
Biological and clinical roles of IL-18 in inflammatory diseases
Nature Reviews Rheumatology (2024)
-
Saharan dust induces NLRP3-dependent inflammatory cytokines in an alveolar air-liquid interface co-culture model
Particle and Fibre Toxicology (2023)
-
The therapeutic potential of targeting regulated non-apoptotic cell death
Nature Reviews Drug Discovery (2023)
-
Production of recombinant human long-acting IL-18 binding protein: inhibitory effect on ulcerative colitis in mice
Applied Microbiology and Biotechnology (2023)
-
5-Hydroxymethylfurfural Ameliorates Allergic Inflammation in HMC-1 Cells by Inactivating NF-κB and MAPK Signaling Pathways
Biochemical Genetics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.