Abstract
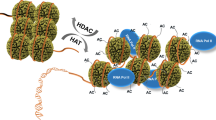
THE yeast transcriptional adaptor1–3, Gcn5p, is a catalytic subunit of a nuclear (type A) histone acetyltransferase linking histone acetylation to gene activation4–6. Here we report that Gcn5p acetylates histones H3 and H4 non-randomly at specific lysines in the amino-terminal domains. Lysine 14 of H3 and lysines 8 and 16 of H4 are highly preferred acetylation sites for Gcn5p. We also demonstrate that lysine 9 is the preferred position of acetylation in newly synthesized yeast H3 in vivo. This finding, along with the fact that lysines 5 and 12 in H4 are predominant acetylation sites during chromatin assembly of many organisms7–11, indicates that Gcn5p acetylates a distinct set of lysines that do not overlap with those sites characteristically used by type B histone acetyltransferase s for histone deposition and chromatin assembly.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Georgakopoulos, T. & Thireos, G. EMBO J. 11, 4145–4152 (1992).
Berger, S. L., Cress, W. D., Cress, A., Triezenberg, S. J. & Guarente, L. Cell 61, 1199–1208 (1990).
Berger, S. L. et al. Cell 70, 251–265 (1992).
Brownell, J. E. et al. Cell 84, 843–851 (1996).
Wolffe, A. P. & Pruss, D. Cell 84, 817–819 (1996).
Brownell, J. E. & Allis, C. D. Curr. Opin. Genet. Dev. 6, 176–184 (1996).
Chicoine, L. G., Schulman, I. G., Richman, R., Cook, R. G. & Allis, C. D. J. Biol. Chem. 261, 1071–1076 (1986).
Sobel, R. E., Cook, R. G. & Allis, C. D. J. Biol. Chem. 269, 18576–18582 (1994).
Munks, R. J., Moore, J., O'Neill, L. P. & Turner, B. M. FEBS Lett. 284, 245–248 (1991).
Turner, B. M., O'Neill, L. P. & Allan, I. M. FEBS Lett. 253, 141–145 (1989).
Thorne, A. W., Kmiciek, D., Mitchelson, K., Sautiere, P. & Crane-Robinson, C. Eur. J. Biochem. 193, 701–713 (1990).
Clarke, D. J., O'Neill, L. P. & Turner, B. M. Biochem. J. 294, 557–561 (1993).
Sobel, R. E., Cook, R. G., Perry, C. A., Annunziato, A. T. & Allis, C. D. Proc. Natl Acad. Sci. USA 92, 1237–1241 (1995).
Candau, R. & Berger, S. L. J. Biol. Chem. 271, 5237–5245 (1996).
Georgakopoulos, T., Gounalaki, N. & Thiros, G. Mol. Gen. Genet. 246, 723–728 (1995).
Horiuchi, J., Silverman, N., Marcus, G. A. & Guarente, L. Mol. Cell. Biol. 15, 1203–1209 (1995).
Yang, X.-J., Ogryzko, V. V., Nishikawa, J.-I., Howard, B. H. & Nakatani, Y. Nature 382, 319–324 (1996).
Edmondson, D. G., Smith, M. M. & Roth, S. Y. Genes Dev. 10, 1247–1259 (1996).
Gorovsky, M. A., Yao, M.-C., Keevert, J. B. & Pleger, G. L. Methods Cell Biol. 9, 311–327 (1975).
Vavra, K. J., Allis, C. D. & Gorovsky, M. A. J. Biol. Chem. 257, 2591–2598 (1982).
Braunstein, M., Sobel, R. E., Allis, C. D., Turner, B. M. & Broach, J. R. Mol. Cell. Biol. 16, 4349–4356 (1996).
Horiuchi, K. & Fujimoto, D. Anal. Biochem. 69, 491–496 (1975).
Brownell, J. E. & Allis, C. D. Proc. Natl Acad. Sci. USA 92, 6364–6368 (1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kuo, MH., Brownell, J., Sobel, R. et al. Transcription-linked acetylation by Gcn5p of histones H3 and H4 at specific lysines. Nature 383, 269–272 (1996). https://doi.org/10.1038/383269a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/383269a0
This article is cited by
-
Lysine acetylation of histone acetyltransferase adaptor protein ADA2 is a mechanism of metabolic control of chromatin modification in plants
Nature Plants (2024)
-
Targeting bromodomain-containing proteins: research advances of drug discovery
Molecular Biomedicine (2023)
-
Adaptive partitioning of a gene locus to the nuclear envelope in Saccharomyces cerevisiae is driven by polymer-polymer phase separation
Nature Communications (2023)
-
A Phagosomally Expressed Gene, rv0428c, of Mycobacterium tuberculosis Demonstrates Acetyl Transferase Activity and Plays a Protective Role Under Stress Conditions
The Protein Journal (2022)
-
HD2-type histone deacetylases: unique regulators of plant development and stress responses
Plant Cell Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.