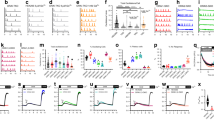
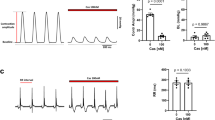
Abstract
CALCIUM ions entering cells through voltage-gated Ca2+ channels initiate rapid release of neurotransmitters and secretion of hormones. Ca2+ currents can be inhibited in many cell types by neurotransmitters acting through G proteins via a membrane-delimited pathway independently of soluble intracellular messengers1–4. Inhibition is typically caused by a positive shift in the voltage dependence and a slowing of channel activation and is relieved by strong depolarization resulting in facilitation of Ca2+currents1,4–6. This pathway regulates the activity of N-type and P/ Q-type Ca2+ channels1,2,7, which are localized in presynaptic terminals8,9 and participate in neurotransmitter release10–13. Synaptic transmission is inhibited by neurotransmitters through this mechanism1,4. G-protein a subunits confer specificity in receptor coupling1–4,14–17, but it is not known whether the Gα or Gβγ subunits are responsible for modulation of Ca2+channels. Here we report that Gβγ subunits can modulate Ca2+ channels. Transfection of Gβγ into cells expressing P/Q-type Ca2+ channels induces modulation like that caused by activation of G protein-coupled receptors, but Gα subunits do not. Similarly, injection or expression of Gβγ subunits in sympathetic ganglion neurons induces facilitation and occludes modulation of N-type channels by noradrenaline, but Gα subunits do not. In both cases, the Gγ subunit is ineffective by itself, but overexpression of exogenous Gβ subunits is sufficient to cause channel modulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hille, B. Trends Neurosci. 17, 531–536 (1994).
Hescheler, J. & Schultz, G. Curr. Opin. Neurobiol. 3, 360–367 (1993).
Wickman, K. D. & Clapham, D. E. Curr. Biol. 5, 278–285 (1995).
Dolphin, A. C. Expl. Physiol. 80, 1–36 (1995).
Marchetti, C., Carbone, E. & Lux, H. D. Pflügers Arch. 406, 104–111 (1986).
Bean, B. P. Nature 340, 153–156 (1989).
Mintz, I. M. & Bean, B. P. Neuron 10, 889–898 (1993).
Robitaille, R., Adler, E. M. & Charlton, M. P. Neuron 5, 773–779 (1990).
Westenbroek, R. E. et al. J. Neurosci. 15, 6403–6418 (1995).
Hirning, L. D. et al. Science 239, 57–61 (1988).
Wheeler, D. B., Randall, A. & Tsien, R. W. Science 264, 107–111 (1994).
Luebke, J. I., Dunlap, K. & Turner, T. J. Neuron 11, 895–902 (1993).
Takahashi, T. & Momiyama, A. Nature 366, 156–158 (1993).
Hescheler, J., Rosenthal, W., Trautwein, W. & Schultz, G. Nature 325, 445–446 (1987).
Wilk-Blaszczak, M. A., Singer, W. D., Gutowski, S., Sternweis, P. C. & Belardetti, F. Neuron 13, 1215–1224 (1994).
Zhu, Y. & Ikeda, S. R. Neuron 13, 657–669 (1994).
Diversé-Pierluissi, M., Goldsmith, P. K. & Dunlap, K. Neuron 14, 191–200 (1995).
Stea, A. et al. Proc. natn. Acad. Sci. U.S.A. 91, 10576–10580 (1994).
Ellis, S. B. et al. Science 241, 1661–1664 (1988).
Clapham, D. E. & Neer, E. J. Nature 365, 403–406 (1993).
Hepler, J. R. & Gilman, A. G. Trends biol. Sci. 17, 383–387 (1992).
Breitwieser, G. E. & Szabo, G. Nature 317, 538–540 (1985).
Pfaffinger, P. J., Martin, J. M., Hunter, D. D., Nathanson, N. M. & Hille, B. Nature 317, 536–538 (1985).
Reuveny, E. et al. Nature 370, 143–146 (1994).
Kofuji, P., Davidson, N. & Lester, H. A. Proc. natn. Acad. Sci. U.S.A. 92, 6542–6546 (1995).
Wickman, K. D. et al. Nature 368, 255–257 (1994).
Chen, J. et al. Science 268, 1166–1169 (1995).
Hockerman, G. H., Johnson, B. D., Scheuer, T. & Catterall, W. A. J. biol. Chem. 270, 22119–22122 (1995).
Bernheim, L., Beech, D. J. & Hille, B. Neuron 6, 859–867 (1991).
Shapiro, M. S., Wollmuth, L. P. & Hille, B. J. Neurosci. 14, 7109–7116 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Herlitze, S., Garcia, D., Mackie, K. et al. Modulation of Ca2+ channels βγ G-protein py subunits. Nature 380, 258–262 (1996). https://doi.org/10.1038/380258a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/380258a0
This article is cited by
-
A sign-inverted receptive field of inhibitory interneurons provides a pathway for ON-OFF interactions in the retina
Nature Communications (2023)
-
Cholinergic deficits selectively boost cortical intratelencephalic control of striatum in male Huntington’s disease model mice
Nature Communications (2023)
-
Involvement of Ca2+ in Signaling Mechanisms Mediating Muscarinic Inhibition of M Currents in Sympathetic Neurons
Cellular and Molecular Neurobiology (2023)
-
Regulation of N-type calcium channels by nociceptin receptors and its possible role in neurological disorders
Molecular Brain (2022)
-
Local modulation by presynaptic receptors controls neuronal communication and behaviour
Nature Reviews Neuroscience (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.